Abstract
Purpose
To investigate developmental competence and neonatal outcomes of nonpronuclear (0PN) zygotes following single vitrified-warmed blastocyst transfers (VBT).
Methods
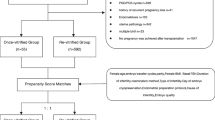
The clinical, laboratorial and neonatal data of 996 patients with ≤ 38 years who underwent blastocyst culture and single VBT were retrospectively analyzed. The pregnancy and neonatal outcomes of VBT were compared between 0PN and 2PN blastocysts using propensity score matching (PSM). Moreover, Day 3 (D3) embryo development and blastocyst formation were compared between 0PN and 2PN zygotes.
Results
There were no significant differences in clinical pregnancy rate (CPR), live birth rate (LBR) and neonatal outcomes of VBT between the 0PN and 2PN blastocysts irrespectively of whether PSM was used. However, early abortion rate (EAR) was higher in blastocysts from 0PN D3 embryos > 10 cells (p < 0.05) before PSM. Moreover, the early developmental competence of 0PN zygotes was different from that of 2PN zygotes presenting higher percentages of D3 embryos ≤ 6 cells (p < 0.01) and > 10 cells (p < 0.01), lower available blastocyst formation rate (ABFR) (p < 0.01) and good-quality blastocyst formation rate (GBFR) (p < 0.01) in D3 embryos with 4–6 cells. ABFR and GBFR increased with cell number when compared among embryos with 4–6 cells, 7–10 cells and > 10 cells, irrespectively of 0PN or 2PN embryos.
Conclusion
The early developmental competence of 0PN zygotes was different from that of 2PN zygotes, but did not influence pregnancy and neonatal outcomes following VBT. ABFR and GBFR increased with cell number, irrespectively of 0PN or 2PN embryos.



Similar content being viewed by others

Data Availability
All relevant data are within the paper.
References
Simopoulou M, Sfakianoudis K, Maziotis E et al (2021) PGT-A: who and when? Α systematic review and network meta-analysis of RCTs. J Assist Reprod Genet 38:1939–1957. https://doi.org/10.1007/s10815-021-02227-9
Zhang WY, von Versen-Höynck F, Kapphahn KI et al (2019) Maternal and neonatal outcomes associated with trophectoderm biopsy. Fertil Steril 112:283-290.e2. https://doi.org/10.1016/j.fertnstert.2019.03.033
Rosenbusch B (2014) The chromosomal constitution of embryos arising from monopronuclear oocytes in programmes of assisted reproduction. Int J Reprod Med 2014:418198. https://doi.org/10.1155/2014/418198
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology (2011) The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 26:1270–1283. https://doi.org/10.1093/humrep/der037
Racowsky C, Stern JE, Gibbons WE et al (2011) National collection of embryo morphology data into society for assisted reproductive technology clinic outcomes reporting system: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril 95:1985–1989. https://doi.org/10.1016/j.fertnstert.2011.02.009
Tian L, Xia L, Liu H et al (2022) Increased blastomere number is associated with higher live birth rate in day 3 embryo transfer. BMC Pregnancy Childbirth 22:198. https://doi.org/10.1186/s12884-022-04521-5
Pons MC, Carrasco B, Parriego M et al (2019) Deconstructing the myth of poor prognosis for fast-cleaving embryos on day 3. Is it time to change the consensus? J Assist Reprod Genet 36:2299–2305. https://doi.org/10.1007/s10815-019-01574-y
Wang J, Diao Z, Fang J et al (2022) The influence of day 3 embryo cell number on the clinical pregnancy and live birth rates of day 5 single blastocyst transfer from frozen embryo transfer cycles. BMC Pregnancy Childbirth 22:980. https://doi.org/10.1186/s12884-022-05337-z
Li M, Lin S, Chen Y et al (2015) Value of transferring embryos that show no evidence of fertilization at the time of fertilization assessment. Fertil Steril 104:607-611.e2. https://doi.org/10.1016/j.fertnstert.2015.05.016
Fu L, Chu D, Zhou W, Li Y (2022) Strictly selected Mono- and non-pronuclear blastocysts could result in appreciable clinical outcomes in IVF cycles. Hum Fertil (Camb) 25:470–477. https://doi.org/10.1080/14647273.2020.1815243
Fu L, Zhou W, Li Y (2021) Development and frozen-thawed transfer of non-pronuclear zygotes-derived embryos in IVF cycles. Eur J Obstet Gynecol Reprod Biol 264:206–211. https://doi.org/10.1016/j.ejogrb.2021.07.033
Yin H, Jiang H, He R et al (2019) Cumulative live birth rate of advanced-age women more than 40 with or without poor ovarian response. Taiwan J Obstet Gynecol 58:201–205. https://doi.org/10.1016/j.tjog.2019.01.006
Gardner DK, Lane M, Stevens J et al (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73:1155–1158. https://doi.org/10.1016/s0015-0282(00)00518-5
Cummins JM, Breen TM, Harrison KL et al (1986) A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf 3:284–295. https://doi.org/10.1007/BF01133388
Yang ST, Shi JX, Gong F et al (2015) Cleavage pattern predicts developmental potential of day 3 human embryos produced by IVF. Reprod Biomed Online 30:625–634. https://doi.org/10.1016/j.rbmo.2015.02.008
Yang S-H, Wu C-H, Chen Y-C et al (2018) Effect of morphokinetics and morphological dynamics of cleavage stage on embryo developmental potential: A time-lapse study. Taiwan J Obstet Gynecol 57:76–82. https://doi.org/10.1016/j.tjog.2017.12.013
Cecchele A, Cermisoni GC, Giacomini E et al (2022) Cellular and molecular nature of fragmentation of human embryos. Int J Mol Sci 23:1349. https://doi.org/10.3390/ijms23031349
Chi H-J, Koo J-J, Choi S-Y et al (2011) Fragmentation of embryos is associated with both necrosis and apoptosis. Fertil Steril 96:187–192. https://doi.org/10.1016/j.fertnstert.2011.04.020
Kong X, Yang S, Gong F et al (2016) The relationship between cell number, division behavior and developmental potential of cleavage stage human embryos: a time-lapse study. PLoS ONE 11:e0153697. https://doi.org/10.1371/journal.pone.0153697
Friedenthal J, Pan S, Gounko D et al (2021) Rate of post-fertilization mitotic activity predicts embryonic competence via next generation sequencing: an analysis of 39,301 cleavage stage embryos. JBRA Assist Reprod 25:586–591. https://doi.org/10.5935/1518-0557.20210051
Luna M, Copperman AB, Duke M et al (2008) Human blastocyst morphological quality is significantly improved in embryos classified as fast on day 3 (> or = 10 cells), bringing into question current embryological dogma. Fertil Steril 89:358–363. https://doi.org/10.1016/j.fertnstert.2007.03.030
Kirkegaard K, Hindkjaer JJ, Ingerslev HJ (2013) Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril 99:738-744.e4. https://doi.org/10.1016/j.fertnstert.2012.11.028
Rubio I, Galán A, Larreategui Z et al (2014) Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the embryoscope. Fertil Steril 102:1287-1294.e5. https://doi.org/10.1016/j.fertnstert.2014.07.738
Kasterstein E, Strassburger D, Komarovsky D et al (2013) The effect of two distinct levels of oxygen concentration on embryo development in a sibling oocyte study. J Assist Reprod Genet 30:1073–1079. https://doi.org/10.1007/s10815-013-0032-z
Yang Y, Dong X, Bai J et al (2022) Faster fertilization and cleavage kinetics reflect competence to achieve a live birth: data from single-embryo transfer cycles. Biomed Res Int 2022:8501362. https://doi.org/10.1155/2022/8501362
Petersen BM, Boel M, Montag M, Gardner DK (2016) Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on day 3. Hum Reprod 31:2231–2244. https://doi.org/10.1093/humrep/dew188
Perry ACF, Asami M, Lam BYH, Yeo GSH (2022) The initiation of mammalian embryonic transcription: to begin at the beginning. Trends Cell Biol S0962–8924(22):00211–00212. https://doi.org/10.1016/j.tcb.2022.08.008
Asami M, Lam BYH, Ma MK et al (2022) Human embryonic genome activation initiates at the one-cell stage. Cell Stem Cell 29:209-216.e4. https://doi.org/10.1016/j.stem.2021.11.012
Feenan K, Herbert M (2006) Can “abnormally” fertilized zygotes give rise to viable embryos? Hum Fertil 9:157–169. https://doi.org/10.1080/14647270600636269
Paz MV, Chiera M, Hovanyecz P et al (2020) Blastocysts derived from 0pn oocytes: genetic and clinical results. JBRA Assist Reprod 24:143–146. https://doi.org/10.5935/1518-0557.20190084
Kobayashi T, Ishikawa H, Ishii K et al (2021) Time-lapse monitoring of fertilized human oocytes focused on the incidence of 0PN embryos in conventional in vitro fertilization cycles. Sci Rep 11:18862. https://doi.org/10.1038/s41598-021-98312-1
Basile N, Morbeck D, García-Velasco J et al (2013) Type of culture media does not affect embryo kinetics: a time-lapse analysis of sibling oocytes. Hum Reprod 28:634–641. https://doi.org/10.1093/humrep/des462
Fragouli E, Munne S, Wells D (2019) The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum Reprod Update 25:15–33. https://doi.org/10.1093/humupd/dmy036
Vega M, Breborowicz A, Moshier EL et al (2014) Blastulation rates decline in a linear fashion from euploid to aneuploid embryos with single versus multiple chromosomal errors. Fertil Steril 102:394–398. https://doi.org/10.1016/j.fertnstert.2014.04.026
Du Y, Guan Y, Li N et al (2023) Is it necessary for young patients with recurrent implantation failure to undergo preimplantation genetic testing for aneuploidy? Front Endocrinol 14:1020055. https://doi.org/10.3389/fendo.2023.1020055
Minasi MG, Colasante A, Riccio T et al (2016) Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod 31:2245–2254. https://doi.org/10.1093/humrep/dew183
Bamford T, Barrie A, Montgomery S et al (2022) Morphological and morphokinetic associations with aneuploidy: a systematic review and meta-analysis. Hum Reprod Update 28:656–686. https://doi.org/10.1093/humupd/dmac022
Zhao H, Yuan P, Chen X et al (2022) The aneuploidy testing of blastocysts developing from 0PN and 1PN zygotes in conventional IVF through TE-biopsy PGT-A and minimally invasive PGT-A. Front Reprod Health 4:966909. https://doi.org/10.3389/frph.2022.966909
Destouni A, Dimitriadou E, Masset H et al (2018) Genome-wide haplotyping embryos developing from 0PN and 1PN zygotes increases transferrable embryos in PGT-M. Hum Reprod 33:2302–2311. https://doi.org/10.1093/humrep/dey325
Li M, Huang J, Zhuang X et al (2021) Obstetric and neonatal outcomes after the transfer of vitrified-warmed blastocysts developing from nonpronuclear and monopronuclear zygotes: a retrospective cohort study. Fertil Steril 115:110–117. https://doi.org/10.1016/j.fertnstert.2020.07.019
Chen C, Li W, Yin M et al (2022) Does the cell number of 0PN embryos on day 3 affect pregnancy and neonatal outcomes following single blastocyst transfer? BMC Pregnancy Childbirth 22:200. https://doi.org/10.1186/s12884-022-04492-7
Castillo CM, Johnstone ED, Horne G et al (2020) Associations of IVF singleton birthweight and gestation with clinical treatment and laboratory factors: a multicentre cohort study. Hum Reprod 35:2860–2870. https://doi.org/10.1093/humrep/deaa244
Baran J, Weres A, Czenczek-Lewandowska E et al (2020) Excessive gestational weight gain: long-term consequences for the child. J Clin Med 9:3795. https://doi.org/10.3390/jcm9123795
Ezoe K, Coticchio G, Takenouchi H et al (2022) Spatiotemporal perturbations of pronuclear breakdown preceding syngamy affect early human embryo development: a retrospective observational study. J Assist Reprod Genet 39:75–84. https://doi.org/10.1007/s10815-021-02335-6
Suzuki R, Yao T, Okada M et al (2023) Direct cleavage during the first mitosis is a sign of abnormal fertilization in cattle. Theriogenology 200:96–105. https://doi.org/10.1016/j.theriogenology.2023.01.028
Uzun KN, Cıncık M, Selam B et al (2021) Comparison of the rates for reaching the blastocyst stage between normal and abnormal pronucleus embryos monitored by a time-lapse system in IVF patients. J Turk Ger Gynecol Assoc 22:120–126. https://doi.org/10.4274/jtgga.galenos.2020.2020.0033
Yin B-L, Hao H-Y, Zhang Y-N et al (2016) Good quality blastocyst from non-/mono-pronuclear zygote may be used for transfer during IVF. Syst Biol Reprod Med 62:139–145. https://doi.org/10.3109/19396368.2015.1137993
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
JZ and HY contributed to the study conception and design. Data collection and analysis were performed by all authors. The first draft of the manuscript was written by JZ and all authors commented on previous versions of the manuscript. HY was involved in finalizing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
This study was approved by the Ethics Committee of the 901st Hospital of the Joint Logistics Support Force of PLA.
Consent to participate
Written informed consent was obtained from all patients.
Consent for publish
All authors agree to transfer the copyright of the current study to the journal upon acceptance.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, J., Wang, C., Cao, Z. et al. Developmental competence and neonatal outcomes of nonpronuclear zygotes following single vitrified-warmed blastocyst transfers using propensity score matching analysis. Arch Gynecol Obstet 309, 295–304 (2024). https://doi.org/10.1007/s00404-023-07235-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07235-x