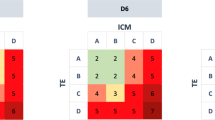
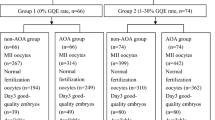
Abstract
Purpose
This study evaluated the relationship between cytoplasmic granulation patterns and the developmental potential of mature sibling oocytes.
Methods
Data from 54 cycles of preimplantation genetic tests for structural rearrangement from July 2019 to June 2022 were analyzed. In total, 564 embryos were cultured using a time-lapse system. Sibling oocytes were divided into four groups based on cytoplasmic granulation patterns: fine granulation (FG) group (n = 177), central granulation (CG) group (n = 183), dispersed granulation (DG) group (n = 161), and uneven granulation (UG) group (n = 43). The CG group was further divided into three groups (grades I, II, and III) based on the tertile of the ratio of central granular distribution area to oocyte area. Fertilization rate, embryo morphokinetics, chromosomal ploidy, and clinical outcomes of the groups were compared.
Results
No significant differences were observed in morphokinetic parameters, fertilization rate, embryo production, blastocyst formation, and aneuploidy rates among the different cytoplasmic-granulation pattern groups. However, embryos derived from CG oocytes showed significantly higher aneuploidy rates in grade III compared to grade I (86.21% vs 61.54%, P = 0.036) or grade II (86.21% vs 56.00%, P = 0.013). Thirty embryos were transferred to the uteri of female patients and the clinical pregnancy and live birth rates did not significantly differ among groups.
Conclusions
Cytoplasmic granulation patterns may not affect embryo fertilization, development speed, and aneuploidy rates. However, a higher grade of CG may be associated with increased aneuploidy rates. Larger sample sizes are required to explore the impact of oocyte cytoplasmic granulation patterns on embryo implantation potential.


Similar content being viewed by others
Data availability
The data are available from the corresponding author on reasonable request.
References
Shen X, Chen D, Ding C, Xu Y, Fu Y, Cai B et al (2022) Evaluating the application value of NGS-based PGT-A by screening cryopreserved MDA products of embryos from PGT-M cycles with known transfer outcomes. J Assist Reprod Genet 39:1323–1331. https://doi.org/10.1007/s10815-022-02447-7
Yan J, Qin Y, Zhao H, Sun Y, Gong F, Li R et al (2021) Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med 385:2047–2058. https://doi.org/10.1056/NEJMoa2103613
Mascarenhas M, Fox SJ, Thompson K, Balen AH (2019) Cumulative live birth rates and perinatal outcomes with the use of time-lapse imaging incubators for embryo culture: a retrospective cohort study of 1882 ART cycles. BJOG 126:280–286. https://doi.org/10.1111/1471-0528.15161
Guo YH, Liu Y, Qi L, Song WY, Jin HX (2021) Can time-lapse incubation and monitoring be beneficial to assisted reproduction technology outcomes? A randomized controlled trial using Day 3 double embryo transfer. Front Physiol 12:794601. https://doi.org/10.3389/fphys.2021.794601
Kovacs P, Matyas S, Forgacs V, Sajgo A, Molnar L, Pribenszky C (2019) Non-invasive embryo evaluation and selection using time-lapse monitoring: results of a randomized controlled study. Eur J Obstet Gynecol Reprod Biol 233:58–63. https://doi.org/10.1016/j.ejogrb.2018.12.011
Fu Y, Chen D, Cai B, Xu Y, Zhu S, Ding C et al (2022) Comparison of two mainstream endometrial preparation regimens in vitrified-warmed embryo transfers after PGT. Reprod Biomed Online 44:239–246. https://doi.org/10.1016/j.rbmo.2021.09.009
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO et al (2010) Standardization of grading embryo morphology. Fertil Steril 94:1152–1153. https://doi.org/10.1016/j.fertnstert.2010.05.042
Gardner DK, Schoolcraft WB (1999) Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol 11:307–311. https://doi.org/10.1097/00001703-199906000-00013
Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M et al (2014) Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod 29:2650–2660. https://doi.org/10.1093/humrep/deu278
Van Blerkom J (2004) Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reprod (Camb Engl) 128:269–280. https://doi.org/10.1530/rep.1.00240
Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML et al (2001) Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 16:909–917. https://doi.org/10.1093/humrep/16.5.909
Hassan-Ali H, Hisham-Saleh A, El-Gezeiry D, Baghdady I, Ismaeil I, Mandelbaum J (1998) Perivitelline space granularity: a sign of human menopausal gonadotrophin overdose in intracytoplasmic sperm injection. Hum Reprod 13:3425–3430. https://doi.org/10.1093/humrep/13.12.3425
Kasapoglu I, Kuspinar G, Saribal S, Turk P, Avcı B, Uncu G (2018) Detrimental effects of endometriosis on oocyte morphology in intracytoplasmic sperm injection cycles: a retrospective cohort study. Gynecol Endocrinol 34:206–211. https://doi.org/10.1080/09513590.2017.1391203
Da Broi MG, Navarro PA (2016) Oxidative stress and oocyte quality: ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility. Cell Tissue Res 364:1–7. https://doi.org/10.1007/s00441-015-2339-9
Dal Canto M, Guglielmo MC, Mignini Renzini M, Fadini R, Moutier C, Merola M et al (2017) Dysmorphic patterns are associated with cytoskeletal alterations in human oocytes. Hum Reprod 32:750–757. https://doi.org/10.1093/humrep/dex041
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM et al (2010) Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol 28:1115–1121. https://doi.org/10.1038/nbt.1686
Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J (2011) The use of morphokinetics as a predictor of embryo implantation. Hum Reprod 26:2658–2671. https://doi.org/10.1093/humrep/der256
Rubio I, Galán A, Larreategui Z, Ayerdi F, Bellver J, Herrero J et al (2014) Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the embryoscope. Fertil Steril 102:1287-1294.e5. https://doi.org/10.1016/j.fertnstert.2014.07.738
Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Ferrero S et al (2008) Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril 90:1692–1700. https://doi.org/10.1016/j.fertnstert.2007.09.024
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R (1998) Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod 13:3431–3433. https://doi.org/10.1093/humrep/13.12.3431
Kahraman S, Yakin K, Dönmez E, Samli H, Bahçe M, Cengiz G et al (2000) Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod 15:2390–2393. https://doi.org/10.1093/humrep/15.11.2390
Tulay P, Arslan H, Buran A, Koprulu Y (2019) Assessment of successful pregnancy using granular oocytes in ICSI treatments. Zygote (Camb Engl) 27:97–100. https://doi.org/10.1017/S096719941900008X
Wilding M, Di Matteo L, D’Andretti S, Montanaro N, Capobianco C, Dale B (2007) An oocyte score for use in assisted reproduction. J Assist Reprod Genet 24:350–358. https://doi.org/10.1007/s10815-007-9143-8
Hu J, Molinari E, Darmon S, Zhang L, Patrizio P, Barad DH et al (2021) Predictive value of cytoplasmic granulation patterns during in vitro fertilization in metaphase II oocytes: part I, Poor-prognosis patients. Fertil Steril 116:431–443. https://doi.org/10.1016/j.fertnstert.2021.02.022
Yi XF, Xi HL, Zhang SL et al (2019) Relationship between the positions of cytoplasmic granulation and the oocytes developmental potential in human. Sci Rep 9:7215. https://doi.org/10.1038/s41598-019-43757-8
Kahraman S, Benkhalifa M, Donmez E, Biricik A, Sertyel S, Findikli N et al (2004) The results of aneuploidy screening in 276 couples undergoing assisted reproductive techniques. Prenat Diagn 24:307–311. https://doi.org/10.1002/pd.842
Acknowledgements
The study was supported by the Guangdong Special Support Program (2019BT02Y276), National Natural Science Foundation of China (81771579), Guangdong Basic and Applied Basic Research Foundation (2021A1515010377), and National Natural Science Foundation of China (32000589). The authors thank Taylor and Francis Editing Services for English language editing.
Funding
This work was supported by grants from the Guangdong Special Support Program (2019BT02Y276), National Natural Science Foundation of China (81771579), Guangdong Basic and Applied Basic Research Foundation (2021A1515010377), and National Natural Science Foundation of China (32000589).
Author information
Authors and Affiliations
Contributions
YW: Conceptualization, Formal Analysis, Project Administration, Resources, Writing–Review and Editing; DC: Data Curation, Investigation, Methodology, Validation, Writing–Original Draft Preparation; BC: Writing–Review and Editing; DH: Data Curation, Formal Analysis; YX: Supervision; CD: Supervision, Writing–Review and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (reference no. [2022] 566, date of approval 27th December 2022). Owing to the retrospective nature of this study, the requirement for informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Chen, D., Cai, B. et al. Effects of different oocyte cytoplasmic granulation patterns on embryo development and euploidy: a sibling oocyte control study. Arch Gynecol Obstet 308, 1593–1603 (2023). https://doi.org/10.1007/s00404-023-07176-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07176-5