Abstract
Introduction
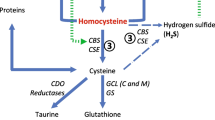
Repeated implantation failure is a common challenge in daily practice. Homocysteine and vitamin B12 have been associated with reproductive processes among patients undergoing in vitro fertilization; however, their involvement in repeated implantation failure has not been assessed. We explored possible associations of serum homocysteine and vitamin B12 with repeated implantation failure.
Material and methods
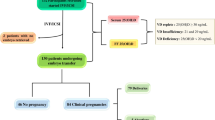
A retrospective analysis of 127 women who underwent ≥ 3 unsuccessful embryo transfers during 2005–2016, at the Fertility and In Vitro Fertilization Unit at Carmel Medical Center. After at least 3 IVF failures serum levels of homocysteine and vitamin B12 were measured.
Results
The mean patient age was 33.5 ± 5.2 years. The mean number of embryo transfers was 4.6 ± 1.5. The mean total cumulative number of embryos transferred was 10.4 ± 5.2. Mean serum levels of homocysteine were 8.6 ± 3.7 µM/L, and of vitamin B12 were 302.5 ± 155.3 pg/ml. Homocysteine levels were within the normal range (< 14 µM/L) in 95.8% of the patients. Yet, the levels of homocysteine correlated with both the number of failed embryo transfers (r = 0.34, p = 0.004) and the total cumulative number of transferred embryos (r = 0.36, p = 0.002).
Conclusions
Our findings suggest an association between serum homocysteine levels and the occurrence of repeated implantation failure, even when homocystein levels were within the normal range. It should be studied whether nutritional supplementation to modulate serum homocysteine levels may improve treatment outcome.


Similar content being viewed by others
Data availability
All data and study materials are available if needed.
Abbreviations
- RIF:
-
Repeated implantation failure
- IVF:
-
In vitro fertilization
References
Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T et al (2014) Recurrent implantation failure: definition and management 14–38
Munné S, Alikani M, Tomkin G, Grifo J, Cohen J (1995) Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril 64(2):382–391
Simon A, Laufer N (2012) Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet 29(11):1227–1239
Medicine PC of the AS for R (2018) The role of immunotherapy in in vitro fertilization: a guideline. Fertil Steril 110(3):387–400
van Kooij RJ, Looman CWN, Habbema JDF, Dorland M, te Velde ER (1996) Age-dependent decrease in embryo implantation rate after in vitro fertilization. Fertil Steril 66(5):769–775
Ocal P, Cift T, Bulut B, Balcan E, Cepni I, Aydogan B et al (2012) Recurrent implantation failure is more frequently seen in female patients with poor prognosis. Int J Fertil Steril 6(2):71–78
Taranissi M, El-Toukhy T, Verlinsky Y (2005) Influence of maternal age on the outcome of PGD for aneuploidy screening in patients with recurrent implantation failure. Reprod Biomed Online 10(5):628–632. https://doi.org/10.1016/S1472-6483(10)61670-7
Basile N, del Nogales M et al (2014) Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil Steril 101(3):699–704. https://doi.org/10.1016/j.fertnstert.2013.12.005
Shi C, Han HJ, Fan LJ, Guan J, Zheng XB, Chen X et al (2018) Diverse endometrial mRNA signatures during the window of implantation in patients with repeated implantation failure. Hum Fertil [Internet]. 21(3):183–194. https://doi.org/10.1080/14647273.2017.1324180
Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F et al (2013) The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 100(3):818–824
Catharine Ross A, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, Stipanuk MH (2014) Chapter 33—Cysteine, taurine and homocysteine. In: Modern nutrition in health and disease 11th Edition. Philadelphia, PA: Lippincott Williams & Wilkins, pp. 447–463
Catharine Ross A, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, Carmel R (2014) Chapter 27—Cobalamin. In: Modern Nutrition In Health And Disease 11th Edition. Philadelphia, PA: Lippincott Williams & Wilkins, pp. 369–389
Desouza C, Keebler M, McNamara DB, Fonseca V (2002) Drugs affecting homocysteine metabolism: impact on cardiovascular risk. Drugs 62(4):605–616
van der Put NMJ, Gabreëls F, Stevens EMB, Smeitink JAM, Trijbels FJM, Eskes TKAB et al (1998) A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet 62(5):1044–1051
D’Elia PQ, Dos Santos AA, Bianco B, Barbosa CP, Christofolini DM, Aoki T (2014) MTHFR polymorphisms C677T and A1298C and associations with IVF outcomes in Brazilian women. Reprod Biomed Online 28(6):733–738. https://doi.org/10.1016/j.rbmo.2014.02.005
Enciso M, Sarasa J, Xanthopoulou L, Bristow S, Bowles M, Fragouli E et al (2016) Polymorphisms in the MTHFR gene influence embryo viability and the incidence of aneuploidy. Hum Genet 135(5):555–568
Clément A, Amar E, Brami C, Clément P, Alvarez S, Jacquesson-Fournols L et al (2022) MTHFR SNPs (methyl tetrahydrofolate reductase, single nucleotide polymorphisms) C677T and A1298C prevalence and serum homocysteine levels in> 2100 hypofertile Caucasian male patients. Biomolecules 12(8):1086
Tara S-S, Ghaemimanesh F, Zarei S, Reihani-Sabet F, Pahlevanzadeh Z, Modarresi MH et al (2015) Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms in male partners of recurrent miscarriage couples. J Reprod Infertil 16(4):193
Hall M, Davidson RJL (1965) Prophylactic folic acid in women with pernicious anaemia pregnant after periods of infertility 599–602
El-Nemr A, Sabatini L, Wilson C, Lower AM, Al-Shawaf T, Grudzinskas JG (1998) Vitamin B12 deficiency and IVF. J Obstet Gynaecol (Lahore) 18(2):192–193
La Vecchia I, Paffoni A, Castiglioni M, Ferrari S, Bortolus R, Ferraris Fusarini C et al (2017) Folate, homocysteine and selected vitamins and minerals status in infertile women. Eur J Contracept Reprod Heal Care 22(1):70–75. https://doi.org/10.1080/13625187.2016.1263292
Paffoni A, Castiglioni M, Ferrari S, La I, Ferraris C, Bettinardi N et al (2018) Homocysteine pathway and in vitro fertilization outcome. Reprod Toxicol 76:12–16. https://doi.org/10.1016/j.reprotox.2017.12.003
Haggarty P, McCallum H, McBain H, Andrews K, Duthie S, McNeill G et al (2006) Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet 367(9521):1513–1519
Boxmeer JC, Steegers-Theunissen RPM, Lindemans J, Wildhagen MF, Martini E, Steegers EAP et al (2008) Homocysteine metabolism in the pre-ovulatory follicle during ovarian stimulation. Hum Reprod 23(11):2570–2576
Parisi F, Rousian M, Koning AHJ, Willemsen SP, Cetin I (2017) Periconceptional maternal one-carbon biomarkers are associated with embryonic development according to the Carnegie stages 32(3):523–530
Szymański W, Kazdepka-Ziemińska A (2003) Effect of homocysteine concentration in follicular fluid on a degree of oocyte maturity. Ginekol Pol 10(74):1392–1396
Berker B, Kaya C, Aytac R, Satiroglu H (2009) Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum Reprod 24(9):2293–2302
Ebisch IMW, Peters WHM, Thomas CMG, Wetzels AMM, Peer PGM, Steegers-Theunissen RPM (2006) Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Hum Reprod 21(7):1725–1733
Aitken RJ, Irvine DS, Wu FC (1991) Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol 164(2):542–551
Jerzak M, Putowski L, Baranowski W (2003) Homocysteine level in ovarian follicular fluid or serum as a predictor of successful fertilization. Ginekol Pol 9(74):949–952
Kralikova M, Melounova J, Crha I, Matejovicova M, Zakova J, Jarkovsky J et al (2011) Intraindividual variability of homocysteine and related thiols concentrations in follicular fluid. J Assist Reprod Genet 28(9):863–868
Menezo YJR, Silvestris E, Dale B, Elder K (2016) Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reprod Biomed Online 33(6):668–683. https://doi.org/10.1016/j.rbmo.2016.09.006
Sato A, Otsu E, Negishi H, Utsunomiya T, Arima T (2007) Aberrant DNA methylation of imprinted loci in superovulated oocytes 22(1):26–35
Menezo Y, Elder K, Benkhalifa M, Dale B (2010) COMMENTARY DNA methylation and gene expression in IVF. Reprod Biomed Online 20(6):709–710. https://doi.org/10.1016/j.rbmo.2010.02.016
Benkhalifa M, Montjean D, Cohen-Bacrie P, Ménézo Y (2010) Imprinting: RNA expression for homocysteine recycling in the human oocyte. Fertil Steril 93(5):1585–1590
Edirimanne VER, Woo CWH, Siow YL, Pierce GN, Xie JY (2007) Homocysteine stimulates NADPH oxidase-mediated superoxide production leading to endothelial dysfunction in rats. Can J Physiol Pharmacol 85(12):1236–1247
Rajani S, Chattopadhyay R, Goswami SK, Ghosh S, Sharma S, Chakravarty B (2012) Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. J Hum Reprod Sci 5(2):187
Huang B, Yang F, Dong X, Zheng Y, Tan H, Ai J et al (2016) Lower limit of antioxidant activity in follicular fluid: relationship to embryo quality in IVF cycle. Int J Clin Exp Med 9(8):16346–16352
Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D et al (2004) Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril 82:1171–1176
Wiener-Megnazi Z, Shiloh H, Avraham L, Lahav-Baratz S, Koifman M, Reznick AZ et al (2011) Oxidative parameters of embryo culture media may predict treatment outcome in in vitro fertilization: A novel applicable tool for improving embryo selection. Fertil Steril 95(3):979–984. https://doi.org/10.1016/j.fertnstert.2010.10.019
Li Y, Gao R, Liu X, Chen X, Liao X, Geng Y et al (2015) Folate deficiency could restrain decidual angiogenesis in pregnant mice. Nutrients 7(8):6425–6445
Gao R, Ding Y, Liu X, Chen X, Wang Y, Long C et al (2012) Effect of folate deficiency on promoter methylation and gene expression of Esr1, Cdh1 and Pgr, and its influence on endometrial receptivity and embryo implantation. Hum Reprod 27(9):2756–2765
Collaboration HLT (2005) Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 82(4):806–812
Qi Q-R, Lechuga TJ, Patel B, Nguyen NA, Yang Y-H, Li Y et al (2020) Enhanced stromal cell CBS-H2S production promotes estrogen-stimulated human endometrial angiogenesis. Endocrinology 161(11):1
Rubini E, Snoek KM, Schoenmakers S, Willemsen SP, Sinclair KD, Rousian M et al (2022) First trimester maternal homocysteine and embryonic and fetal growth: the Rotterdam periconception cohort. Nutrients 14(6):1129
Robinson K, Mayer E, Miller D, Green R, Lente F, Gupta A et al (1995) Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation 92:2825–2830
Iqbal MP (2006) Hyperhomocysteinemia and coronary artery disease in Pakistan. J Pak Med Assoc 56(6):282–284
Jacques PF, Rosenberg IH, Rogers G, Selhub J, Bowman BA, Gunter EW et al (1999) Serum total homocysteine concentrations in adolescent and adult Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr 69(3):482–489
Funding
The study was not founded.
Author information
Authors and Affiliations
Contributions
NF-M: Acquisition of data, Statistical analysis. HG: Critical revision of the manuscript for important intellectual content. IF: Analysis and Interpretation of data. SL-B: Analysis and Interpretation of data. IB-S: Supervision, Critical revision of the manuscript for important intellectual content. ZW-M: Conception and design of the manuscript, Critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this article.
Ethical approval
The study was approved by the institutional ethical review board (IRB).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manzur, N.F., Gluska, H., Feferkorn, I. et al. Homocysteine serum levels correlate with the number of failed IVF cycles even when within normal range. Arch Gynecol Obstet 307, 1975–1982 (2023). https://doi.org/10.1007/s00404-023-06972-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-06972-3