Abstract
Background
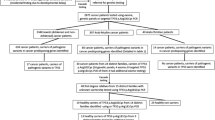
This paper evaluated the clinical utility of massively parallel sequencing-based non-invasive prenatal testing (NIPT) for detecting trisomy 21 (T21), T18, T13, sex chromosome aneuploidies (SCA), and rare chromosome aneuploidies (RCA) among the data collected by a clinical laboratory in southern China.
Methods
In a 3-year period between January 2017 and December 2019, over 40,000 pregnant women underwent NIPT clinical screening test for fetal T21, T18, T13, SCA, and RCA in our laboratory. NIPT samples were processed using the NextSeq CN500 platform. The positive results were confirmed by karyotyping, and chromosomal microarray analysis (CMA) or copy number variants (CNV) sequencing. Details of the pregnancy outcomes were collected via telephone interview.
Results
NIPT results were available for 41,819 cases; 691 positive cases were reported. The overall sensitivity for detection of T21, T18, T13, SCA, and RCA was 99.21, 100.00, 100.00, 98.55, and 100.00%, and the specificity was 99.95, 99.94, 99.98, 99.69, and 99.92%, respectively. The positive predictive values (PPVs) for detection of T21, T18, T13, SCA, and RCA were 85.62, 45.24, 40.00, 34.17, and 13.51%, respectively, and those for detection of 45,X, 47,XXY, 47,XXX, 47,XYY, and 46,XY(delX) 20.00, 59.18, 28.95, 61.54, and 25.00%, respectively. Regarding pregnancy outcomes, 92.38% of the pregnancies with confirmed aneuploidies were terminated, and 91.20% of those identified as having a false-positive result were carried to term. Among 252 unconfirmed cases, 24.60% of the pregnancies were terminated and 38.10% carried to term, while 37.30% declined interview.
Conclusions
NIPT is widely used to screen fetal aneuploidies based on its high sensitivity and specificity. However, in this study, the PPVs of NIPT in terms of detecting T18, T13, XO, XXX and RCA were < 50%. In addition, more than one-third of NIPT-positive women did not accept invasive prenatal diagnosis. Confirmatory diagnosis is strongly recommended for women with positive NIPT outcomes before any further decision is made.



Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lo YM, Corbetta N, Chamberlain PF et al (1997) Presence of fetal DNA in maternal plasma and serum. Lancet 350(9076):485–487
Chiu RW, Chan KC, Gao Y et al (2008) Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A 105(51):20458–20463
Bianchi DW, Rava RP, Sehnert AJ (2014) DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med 371(6):578
Norton ME, Jacobsson B, Swamy GK et al (2015) Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med 372(17):1589–1597
Gregg AR, Skotko BG, Benkendorf JL et al (2016) Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med 18(10):1056–1065
Committee Opinion No (2015) 640: Cell-Free DNA Screening For Fetal Aneuploidy. ObstetGynecol 126(3):e31–e37
van der Meij KRM, Sistermans EA, Macville MVE et al (2019) TRIDENT-2: National implementation of genome-wide non-invasive prenatal testing as a first-tier screening test in the Netherlands. Am J Hum Genet 105(6):1091–1101
Guy C, Haji-Sheikhi F, Rowland CM et al (2019) Prenatal cell-free DNA screening for fetal aneuploidy in pregnant women at average or high risk: Results from a large US clinical laboratory. Mol Genet Genomic Med 7(3):e545
La Verde M, De Falco L, Torella A et al (2021) Performance of cell-free DNA sequencing-based non-invasive prenatal testing: experience on 36,456 singleton and multiple pregnancies. BMC Med Genomics 14(1):93
Soster E, Boomer T, Hicks S et al (2021) Three years of clinical experience with a genome-wide cfDNA screening test for aneuploidies and copy-number variants. Genet Med 23(7):1349–1355
Li Q, Deng D (2017) New medical risks affecting obstetrics after implementation of the two-child policy in China. Front Med 11(4):570–575
Xue Y, Zhao G, Li H et al (2019) Non-invasive prenatal testing to detect chromosome aneuploidies in 57,204 pregnancies. Mol Cytogenet 12:29
Liang D, Cram DS, Tan H et al (2019) Clinical utility of noninvasive prenatal screening for expanded chromosome disease syndromes. Genet Med 21(9):1998–2006
Chen Y, Yu Q, Mao X, Lei W, He M, Lu W (2019) Noninvasive prenatal testing for chromosome aneuploidies and subchromosomal microdeletions/ microduplications in a cohort of 42,910 single pregnancies with different clinical features. Hum Genomics 13(1):60
Xu L, Huang H, Lin N et al (2020) Non-invasive cell-free fetal DNA testing for aneuploidy: multicenter study of 31 515 singleton pregnancies in southeastern China. Ultrasound Obstet Gynecol 55(2):242–247
Liu S, Yang F, Chang Q, Jia B, Xu Y, Wu R, Li L, Chen W, Yin A, Huang F, Feng S, Li F (2022) Positive predictive value estimates for noninvasive prenatal testing from data of a prenatal diagnosis laboratory and literature review. Mol Cytogenet 15(1):29
Demko Z, Prigmore B, Benn P (2022) A critical evaluation of validation and clinical experience studies in non-invasive prenatal testing for trisomies 21, 18, and 13 and monosomy X. J Clin Med 11(16):4760
Gadsbøll K, Petersen OB, Gatinois V et al (2021) Current use of noninvasive prenatal testing in Europe, Australia and the USA: A graphical presentation. Acta ObstetGynecol Scand. 99(6):722–730
Lüthgens K, Grati FR, Sinzel M, Häbig K, Kagan KO (2020) Confirmation rate of cell free DNA screening for sex chromosomal abnormalities according to the method of confirmatory testing. PrenatDiagn. 41(10):1258–1263
Wang Y, Chen Y, Tian F et al (2014) Maternal mosaicism is a significant contributor to discordant sex chromosomal aneuploidies associated with noninvasive prenatal testing. Clin Chem 60(1):251–259
Wang S, Huang S, Ma L et al (2015) Maternal X chromosome copy number variations are associated with discordant fetal sex chromosome aneuploidies detected by noninvasive prenatal testing. Clin Chim Acta 444:113–116
Russell LM, Strike P, Browne CE, Jacobs PA (2007) X chromosome loss and ageing. Cytogenet Genome Res 116(3):181–185
Chen Y, Lai Y, Xu F et al (2021) The application of expanded noninvasive prenatal screening for genome-wide chromosomal abnormalities and genetic counseling. J Matern Fetal Neonatal Med 34(16):2710–2716
Screening for Fetal Chromosomal Abnormalities (2020) ACOG practice bulletin summary, number 226. Obstet Gynecol 136(4):859–867
Zhou Q, Zhu ZP, Zhang B, Yu B, Cai ZM, Yuan P (2019) Clinical features and pregnancy outcomes of women with abnormal cell-free fetal DNA test results. Ann Transl Med 7(14):317
Acknowledgements
The authors thank all of the project participants for their contributions. We also thank Textcheck (http://www.textcheck.com) for language editing.
Funding
The study was supported by Science and Technology Planning Project of Guangzhou, China (201604020104).
Author information
Authors and Affiliations
Contributions
FL, QC and XY conceived the analysis. FL drafted the manuscript. SL and YX reviewed NIPT results. RW, WC, and FH performed the validation experiments. QC, FY, BJ, LL and AY performed clinical diagnosis and communication with patients. FL followed the pregnancy outcome. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Ethical approval
Written informed consent was obtained from all subjects and their legal guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Chang, Q., Yang, F. et al. Non-invasive prenatal test findings in 41,819 pregnant women: results from a clinical laboratory in southern China. Arch Gynecol Obstet 308, 787–795 (2023). https://doi.org/10.1007/s00404-022-06908-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06908-3