Abstract
Purpose
The distribution of human papillomavirus (HPV) varies geographically, and each country is making its screening and vaccination program. This study questioned the need for colposcopy for HPV types other than HPV 16 and 18, and the need for cytology incorporated into HPV testing.
Methods
1043 consecutive patients referred for colposcopy are included in this retrospective study. Logistic regression analysis, ANOVA, and Pearson’s correlation were used for statistical analysis. The value of p < 0.05 was considered statistically significant.
Results
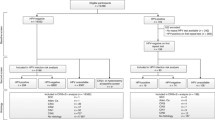
HPV 16 was the most common HPV type referred, followed by HPV 18, 52, 51, and 31, respectively. HPV 16 tends to be positive in younger patients than other HPV types (p < 0.05). Only HPV 16 (OR: 1.41, 1.06–1.88 95% CI) and HPV 33 (OR: 2.23; 1.06–4.64 95% CI) (p < 0.05) had significant prediction for CIN 2 + lesions. In patients with only a cytological abnormality, cytological abnormality with single other high-risk (hr) HPV (without HPV 16 or 18) or double other hrHPV positivity but without HPV 16 and 18 infections, we detected 159 (19%) CIN 2 + lesions.
Conclusion
HPV 33 may be implemented in hrHPV screening protocols for direct colposcopy referral as well as HPV 16 and HPV 18 in specific regions. If we had opted for HPV-based screening only for HPV 16 and 18 without cytology, 19% of all CIN 2 + lesions would have been missed. HPV-based screening only with HPV 16 and 18 may not be feasible. Nonavalent vaccines should be considered for the vaccination of specific populations.

Similar content being viewed by others
Availability of data and material
Data supporting this study’s findings are available from the corresponding author (BT) upon reasonable request and with permission from the Institutional Review Board of Health Sciences University Samsun Research and Training Hospital.
References
Zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350
Vink MA, Bogaards JA, van Kemenade FJ, de Melker HE, Meijer CJ, Berkhof J (2013) Clinical progression of high-grade cervical intraepithelial neoplasia: estimating the time to preclinical cervical cancer from doubly censored national registry data. Am J Epidemiol 178:1161–1169
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S (2007) Human papillomavirus and cervical cancer. Lancet 370:890–907
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 Countries. CA: A Cancer J Clin 71:209–249
Levi F, Lucchini F, Negri E, Franceschi S, la Vecchia C (2000) Cervical cancer mortality in young women in Europe: patterns and trends. Eur J Cancer 36:2266–2271
Franco EL, Duarte-Franco E, Ferenczy A (2001) Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ 164:1017–1025
Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K et al (2007) Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 357:1589–1597
Anttila A, Kotaniemi-Talonen L, Leinonen M, Hakama M, Laurila P, Tarkkanen J et al (2010) Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 340:c1804
Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DA et al (2012) Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol 13:78–88
Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M et al (2014) Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 383:524–532
Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA et al (2017) Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev 8:CD008587
Castle PE, Stoler MH, Solomon D, Schiffman M (2007) The relationship of community biopsy-diagnosed cervical intraepithelial neoplasia grade 2 to the quality control pathology-reviewed diagnoses: an ALTS report. Am J Clin Pathol 127:805–815
Carreon JD, Sherman ME, Guillen D, Solomon D, Herrero R, Jeronimo J et al (2007) CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol 26:441–446
Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F et al (2020) 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 24:102–131
Gultekin M, Zayifoglu Karaca M, Kucukyildiz I, Dundar S, Boztas G, Semra Turan H et al (2018) Initial results of population based cervical cancer screening program using HPV testing in one million Turkish women. Int J Cancer 142:1952–1958
Rijkaart DC, Berkhof J, van Kemenade FJ, Coupe VM, Hesselink AT, Rozendaal L et al (2012) Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int J Cancer 130:602–610
Miralpeix E, Genovés J, Maria Solé-Sedeño J, Mancebo G, Lloveras B, Bellosillo B et al (2017) Usefulness of p16INK4a staining for managing histological high-grade squamous intraepithelial cervical lesions. Mod Pathol 30:304–310
Vitale SG, Valenti G, Rapisarda AMC, Cali I, Marilli I, Zigarelli M et al (2016) P16INK4a as a progression/regression tumour marker in LSIL cervix lesions: our clinical experience. Eur J Gynaecol Oncol 37:685–688
Cancer Genome Atlas Research N, Albert Einstein College of M, Analytical Biological S, Barretos Cancer H, Baylor College of M, Beckman Research Institute of City of H et al (2017) Integrated genomic and molecular characterization of cervical cancer. Nature 543:378–384
Demarco M, Lorey TS, Fetterman B, Cheung LC, Guido RS, Wentzensen N et al (2017) Risks of CIN 2+, CIN 3+, and cancer by cytology and human papillomavirus status: the foundation of risk-based cervical screening guidelines. J Low Genit Tract Dis 21:261–267
Xing B, Guo J, Sheng Y, Wu G, Zhao Y (2020) Human papillomavirus-negative cervical cancer: a comprehensive review. Front Oncol 10:606335
Pirog EC, Lloveras B, Molijn A, Tous S, Guimerà N, Alejo M et al (2014) HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol 27:1559–1567
Jenkins D, Molijn A, Kazem S, Pirog EC, Alemany L, de Sanjose S et al (2020) Molecular and pathological basis of HPV-negative cervical adenocarcinoma seen in a global study. Int J Cancer 147:2526–2536
Guimera N, Lloveras B, Lindeman J, Alemany L, van de Sandt M, Alejo M et al (2013) The occasional role of low-risk human papillomaviruses 6, 11, 42, 44, and 70 in anogenital carcinoma defined by laser capture microdissection/PCR methodology: results from a global study. Am J Surg Pathol 37:1299–1310
Snijders PJ, Hogewoning CJ, Hesselink AT, Berkhof J, Voorhorst FJ, Bleeker MC et al (2006) Determination of viral load thresholds in cervical scrapings to rule out CIN 3 in HPV 16, 18, 31 and 33-positive women with normal cytology. Int J Cancer 119:1102–1107
Moberg M, Gustavsson I, Gyllensten U (2004) Type-specific associations of human papillomavirus load with risk of developing cervical carcinoma in situ. Int J Cancer 112:854–859
Long W, Yang Z, Li X, Chen M, Liu J, Zhang Y et al (2018) HPV-16, HPV-58, and HPV-33 are the most carcinogenic HPV genotypes in Southwestern China and their viral loads are associated with severity of premalignant lesions in the cervix. Virology Journal 15:94
Karadza M, Zidovec Lepej S, Planinic A, Grgic I, Corusic A, Planinic P et al (2021) Distribution of human papillomavirus genotypes in women with high-grade cervical intraepithelial lesions and cervical carcinoma and analysis of human papillomavirus-16 genomic variants. Croat Med J 62:68–79
Chattopadhyay K (2011) A comprehensive review on host genetic susceptibility to human papillomavirus infection and progression to cervical cancer. Indian J Hum Genet 17:132–144
Porras C, Rodriguez AC, Hildesheim A, Herrero R, Gonzalez P, Wacholder S et al (2009) Human papillomavirus types by age in cervical cancer precursors: predominance of human papillomavirus 16 in young women. Cancer Epidemiol Biomarkers Prev 18:863–865
Sigurdsson K, Taddeo FJ, Benediktsdottir KR, Olafsdottir K, Sigvaldason H, Oddsson K et al (2007) HPV genotypes in CIN 2–3 lesions and cervical cancer: a population-based study. Int J Cancer 121:2682–2687
Schmitt M, Depuydt C, Benoy I, Bogers J, Antoine J, Arbyn M et al (2013) Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol 51:1458–1464
Pista A, Oliveira A, Verdasca N, Ribeiro F (2011) Single and multiple human papillomavirus infections in cervical abnormalities in Portuguese women. Clin Microbiol Infect 17:941–946
Cuschieri KS, Cubie HA, Whitley MW, Seagar AL, Arends MJ, Moore C et al (2004) Multiple high risk HPV infections are common in cervical neoplasia and young women in a cervical screening population. J Clin Pathol 57:68–72
Funding
The author declares that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
As the only author, BT examined the patients, did the colposcopies, took records, applied to the ethics committee, and prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author has no relevant financial or non-financial interests to disclose.
Ethical statement
The work has been approved by Health Sciences University Samsun Research and Training Hospital Institutional Review Board (IRB) and Ethics Committee with the approval number TUEK 67-2019BADK/13-101.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tatar, B. Incorporating HPV 33 and cytology into HPV 16/18 screening may be feasible. A cross-sectional study. Arch Gynecol Obstet 308, 183–191 (2023). https://doi.org/10.1007/s00404-022-06876-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06876-8