Abstract
Purpose
A new POP consisting of 4 mg drospirenone (DRSP) for 24 days with a 4-day hormone-free interval was developed to improve bleeding predictability during POP use. The aim of this study was to evaluate the effect on bleeding patterns during use of this oral contraceptive (OC) in comparison with previous menstrual cycles before the start of OC use.
Methods
This is a pilot, prospective trial. A diary was used to collect information about daily bleeding and pelvic pain before and during treatment. During OC use, women were categorised as having (1) unscheduled bleeding or spotting days (UB), (2) scheduled bleeding or spotting days (SB) and (3) absence of bleeding/spotting (AB). SF-36 and FSFI questionnaires were used to quantify health-related quality of life and the quality of sexual life in sexually active participants.
Results
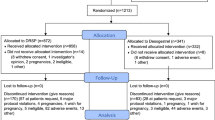
Eighteen out of twenty-five (72%) women completed the entire follow-up. Women with UB (44.4%) were older at inclusion (p < 0.001) and had higher BMIs (p = 0.02) than those with AB (22.2%) or SB (33.4%). Women recorded a significant reduction of menstrual flow intensity during OC use (p < 0.0001). Those with UB also experienced a significant reduction of menstrual pain intensity (p = 0.006). Women with SB during OC use had a longer baseline cycle than those who reported UB during OC use (p = 0.008). Satisfaction with this OC was very high (8.4 ± 2.2 points) with no modification in SF-36 and FSFI values.
Conclusion
A DRSP-only pill is a good OC option for women with contraindications to oestrogen use. Features of the menstrual cycle before the start of OC use may be used to predict associated changes in bleeding patterns.

Similar content being viewed by others
Data availability
Data available on request due to privacy/ethical restrictions.
References
World Health Organization (2015) Medical eligibility criteria for contraceptive use, 5th edn. WHO, Geneva
Grandi G, Cagnacci A, Volpe A (2014) Pharmacokinetic evaluation of desogestrel as a female contraceptive. Expert Opin Drug Metab Toxicol 10(1):1–10
Kovacs G (1996) Progestogen-only pills and bleeding disturbances. Hum Reprod 11(2):20–23
Del Savio MC, De Fata R, Facchinetti F, Grandi G (2020) Drospirenone 4 mg-only pill (DOP) in 24+4 regimen: a new option for oral contraception. Expert Rev Clin Pharmacol 13(7):685–694
Palacios S, Regidor PA, Colli E, Skouby SO, Apter D, Roemer T, Egarter C, Nappi RE, Skřivánek A, Jakimiuk AJ, Weyers S, Ács N, Elia D, Gemzell Danielsson K, Bitzer J (2020) Oestrogen-free oral contraception with a 4 mg drospirenone-only pill: new data and a review of the literature. Eur J Contracept Reprod Health Care 25(3):221–227
Palacios S, Colli E, Regidor PA (2020) Bleeding profile of women using a drospirenone-only pill 4 mg over nine cycles in comparison with desogestrel 0.075 mg. PLoS ONE 15(6):e0231856
Palacios S, Colli E, Regidor PA (2019) A multicenter, double-blind, randomized trial on the bleeding profile of a drospirenone-only pill 4 mg over nine cycles in comparison with desogestrel 0.075 mg. Arch Gynecol Obstet 300(6):1805–1812
Reed BG, Carr BR (2018) The normal menstrual cycle and the control of ovulation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (eds) Endotext [Internet]. MDText.com, Inc, South Dartmouth (MA), p 2000
Creinin MD, Vieira CS, Westhoff CL, Mansour DJA (2022) Recommendations for standardization of bleeding data analyses in contraceptive studies. Contraception 112:14–22
Archer DF, Ahrendt HJ, Drouin D (2015) Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception 92(5):439–444
Regidor PA, Palacios S, Colli E (2022) Bleeding profile of women with cardiovascular risk factors using a drospirenone only pill with 4 mg over nine cycles compared to desogestrel 0.075 mg. Gynecol Endocrinol 38(4):333–338
Both S, Lew-Starowicz M, Luria M, Sartorius G, Maseroli E, Tripodi F, Lowenstein L, Nappi RE, Corona G, Reisman Y, Vignozzi L (2019) Hormonal contraception and female sexuality: position statements from the European society of sexual medicine (ESSM). J Sex Med 16(11):1681–1695
Zimmerman Y, Eijkemans MJ, Coelingh Bennink HJ, Blankenstein MA, Fauser BC (2014) The effect of combined oral contraception on testosterone levels in healthy women: a systematic review and meta-analysis. Hum Reprod Update 20(1):76–105
Regidor PA, Colli E, Palacios S (2021) Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol 37(12):1121–1127
Fruzzetti F, Perini D, Fornaciari L, Russo M, Bucci F, Gadducci A (2016) Discontinuation of modern hormonal contraceptives: an Italian survey. Eur J Contracept Reprod Health Care 21(6):449–454
Nappi RE, Kaunitz AM, Bitzer J (2016) Extended regimen combined oral contraception: a review of evolving concepts and acceptance by women and clinicians. Eur J Contracept Reprod Health Care 21(2):106–115
Kimble T, Burke AE, Barnhart KT, Archer DF, Colli E, Westhoff CL (2020) A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contraception 2:100020
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
GG: concept and design, data analysis, interpretation, manuscript draft, final approval. MCDS: manuscript revision, final approval. CM: study execution, final approval. FF: interpretation, data analysis, manuscript revision, final approval.
Corresponding author
Ethics declarations
Conflict of interest
G. Grandi received honoraria for sponsored lectures and participation in advisory boards from Bayer AG, Italfarmaco, Theramex, Organon, Gedeon Richter and Exeltis, not related to this manuscript. Other authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grandi, G., Del Savio, M.C., Melotti, C. et al. Drospirenone 4 mg in a 24 + 4 regimen in women with contraindications to oestrogen use for contraception: bleeding patterns according to previous menstrual characteristics. Arch Gynecol Obstet 307, 873–879 (2023). https://doi.org/10.1007/s00404-022-06853-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06853-1