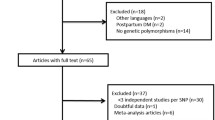
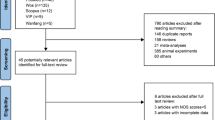
Abstract
Purpose
Previous epidemiological data linking the C677T and A1298C MTHFR polymorphisms to gestational diabetes risk have been mixed and controversial. Therefore, we conducted this meta-analysis to derive a more precise estimation of the relationship between MTHFR polymorphisms and this pregnancy disorder.
Methods
A systematic literature search for original epidemiological studies was performed in the CNKI, WanFang, Cochrane Library, PubMed, and Web of Science databases. R language-based programs were employed for all statistical analyses. Odds ratios and corresponding 95% confidence intervals were calculated to estimate the effects of the variant allele on gestational diabetes risk.
Results
A summary of the estimates for the C677T polymorphism showed that the exposure cohorts were prone to gestational diabetes by a greater magnitude than the control groups. Further subgroup analysis by ethnicity showed that the Asians carrying the variant T allele were more susceptible to this pregnancy disorder. However, the pathogenic effect was not evident in the non-Asian subgroup. For the A1298C polymorphism, no statistical significance could be detected.
Conclusion
This meta-analysis suggests that the T allele of the MTHFR gene C677T polymorphism tends to increase gestational diabetes susceptibility, especially for Asians. However, the A1298C polymorphism is not associated with an increased risk of this crippling pregnancy disorder.




Similar content being viewed by others
Availability of data and material
The corresponding author has all the data and materials.
References
Diagnosis and classification of diabetes mellitus (2014) Diabetes Care 37 (Supplement 1):S81 https://doi.org/10.2337/dc14-S081
Kunasegaran T, Balasubramaniam V, Arasoo VJT, Palanisamy UD, Ramadas A (2021) Gestational diabetes mellitus in southeast asia: a scoping review. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18031272
Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C (2003) Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol 158(12):1148–1153. https://doi.org/10.1093/aje/kwg273
Bellamy L, Casas J-P, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. The Lancet 373(9677):1773–1779. https://doi.org/10.1016/S0140-6736(09)60731-5
ACOG practice bulletin No. 190: gestational diabetes mellitus (2018) Obstet Gynecol 131 (2):e49–e64 https://doi.org/10.1097/aog.0000000000002501
Schmidt MI, Duncan BB, Reichelt AJ, Branchtein L, Matos MC, Costa e Forti A, Spichler ER, Pousada JM, Teixeira MM, Yamashita T (2001) Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care 24(7):1151–1155. https://doi.org/10.2337/diacare.24.7.1151
O’Sullivan EP, Avalos G, O’Reilly M, Dennedy MC, Gaffney G, Dunne F (2011) Atlantic diabetes in pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia 54(7):1670–1675. https://doi.org/10.1007/s00125-011-2150-4
Johns EC, Denison FC, Norman JE, Reynolds RM (2018) Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab 29(11):743–754. https://doi.org/10.1016/j.tem.2018.09.004
Li M, Li S, Chavarro JE, Gaskins AJ, Ley SH, Hinkle SN, Wang X, Ding M, Bell G, Bjerregaard AA, Olsen SF, Mills JL, Hu FB, Zhang C (2019) Prepregnancy habitual intakes of total, supplemental, and food folate and risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. https://doi.org/10.2337/dc18-2198
Cheng G, Sha T, Gao X, He Q, Wu X, Tian Q, Yang F, Tang C, Wu X, Xie Q, Yan Y (2019) The associations between the duration of folic acid supplementation, gestational diabetes mellitus, and adverse birth outcomes based on a birth cohort. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16224511
Yang Y, Cai Z, Zhang J (2021) Association between maternal folate status and gestational diabetes mellitus. Food Sci Nutr. https://doi.org/10.1002/fsn3.2173
Jankovic-Karasoulos T, Furness DL, Leemaqz SY, Dekker GA, Grzeskowiak LE, Grieger JA, Andraweera PH, McCullough D, McAninch D, McCowan LM, Bianco-Miotto T, Roberts CT (2021) Maternal folate, one-carbon metabolism and pregnancy outcomes. Matern Child Nutr 17(1):e13064. https://doi.org/10.1111/mcn.13064
Li S, Hou Y, Yan X, Wang Y, Shi C, Wu X, Liu H, Zhang L, Zhang X, Liu J, Zhang M, Zhang Q, Tang N (2019) Joint effects of folate and vitamin B12 imbalance with maternal characteristics on gestational diabetes mellitus. J Diabetes 11(9):744–751. https://doi.org/10.1111/1753-0407.12899
Chen X, Zhang Y, Chen H, Jiang Y, Wang Y, Wang D, Li M, Dou Y, Sun X, Huang G, Yan W (2021) Association of maternal folate and vitamin B12 in early pregnancy with gestational diabetes mellitus: a prospective cohort study. Diabetes Care 44(1):217. https://doi.org/10.2337/dc20-1607
Weisberg I, Tran P, Christensen B, Sibani S, Rozen R (1998) A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64(3):169–172. https://doi.org/10.1006/mgme.1998.2714
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJH, den Heijer M, Kluijtmans LAJ, van den Heuve LP, Rozen R (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10(1):111–113. https://doi.org/10.1038/ng0595-111
Chen Y, Yang F, Jia T, Liang B, Kong L, Shen J (2019) Association between MTHFR and TCF7L2 polymorphisms and risk of gestational diabetes mellitus. Matern Child Health Care China 34(20):4728–4730
Cheng L, Wang D, Ye G, Yuan C, Peng J (2016) Association between homocystine and C677T polymorphism with gestational diabetes mellitus. Int J Lab Med 37(6):736–737. https://doi.org/10.3969/j.issn.1673-4130.2016.06.007
Fang J, Yang Y, Lu W (2020) Correlation analysis of MTHFR C677T polymorphism with serum vitamin A, vitamin E and pregnancy outcome in patients with gestational diabetes mellitus. Chin J Birth Health Hered 28(6):666–670. https://doi.org/10.13404/j.cnki.cjbhh.2020.06.004
Franzago M, Fraticelli F, Marchetti D, Celentano C, Liberati M, Stuppia L, Vitacolonna E (2018) Nutrigenetic variants and cardio-metabolic risk in women with or without gestational diabetes. Diabetes Res Clin Pract 137:64–71. https://doi.org/10.1016/j.diabres.2018.01.001
Guan H, Yu T (2018) The relationship between the polymorphism of MTHFR and β-CBS with GDM. China Health Stand Manag 9(3):140–141. https://doi.org/10.3969/j.issn.1674-9316.2018.03.077
Khan IA, Shaik NA, Kamineni V, Jahan P, Hasan Q, Rao P (2015) Evaluation of gestational diabetes mellitus risk in south indian women based on MTHFR (C677T) and FVL (G1691A) mutations. Front Pediatr 3:34. https://doi.org/10.3389/fped.2015.00034
Li S, Zhao R, Zheng J (2019) Association of C677T polymorphism of MTHFR gene and overweight/obesity with the risk of gestational diabetes mellitus. Chin J Front Med Sci 11(10):114–117. https://doi.org/10.12037/YXQY.2019.10-25
Liu PJ, Liu Y, Ma L, Yao AM, Chen XY, Hou YX, Wu LP, Xia LY (2020) Associations between gestational diabetes mellitus risk and folate status in early pregnancy and MTHFR C677T polymorphisms in Chinese women. Diabetes Metab Syndr Obes 13:1499–1507. https://doi.org/10.2147/DMSO.S250279
Ni F, Zheng J, Xu X, Zheng X, Sun C, Huang Z, Zhang H (2020) Correlation between serum homocysteine, folic acid, vitamin B12 and MTHFR gene C677T polymorphism and gestational diabetes mellitus. China Modern Doctor 58(12):68–71
Xing W, Fei P, Lin L (2019) Effect of MTHFR C677 polymorphism and overweight/obesity on the risk of GDM. Chin J Coal Ind Med 22(2):113–117
Yang M (2016) Association of plasma homocysteine, leptin and related gene polymorphisms and gestational diabetes mellitus. Huazhong University of Science and Technology, Wuhan, Hubei Province, China
Zhang Y, Yu T, Guan H (2018) Association between MTHFR gene A1298C polymorphism and gestational diabetes mellitus risk. China Health Care Nutr 28(5):264. https://doi.org/10.3969/j.issn.1004-7484.2018.05.391
Dias S, Adam S, Rheeder P, Pheiffer C (2021) No association between ADIPOQ or MTHFR polymorphisms and gestational diabetes mellitus in South African women. Diabetes Metab Syndr Obes 14:791–800. https://doi.org/10.2147/dmso.S294328
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748. https://doi.org/10.1093/jnci/22.4.719
Galbraith RF (1988) A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 7(8):889–894. https://doi.org/10.1002/sim.4780070807
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics. https://doi.org/10.2307/2533446
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Pu J, Romanelli R, Zhao B, Chung S, Nimbal V, Palaniappan L (2014) Higher prevalence of insulin resistance among asian americans despite lower body mass index. Circulation 130(suppl_2):A19858–A19858
Raji A, Seely EW, Arky RA, Simonson DC (2001) Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab 86(11):5366–5371. https://doi.org/10.1210/jcem.86.11.7992
Raygor V, Abbasi F, Lazzeroni LC, Kim S, Ingelsson E, Reaven GM, Knowles JW (2019) Impact of race/ethnicity on insulin resistance and hypertriglyceridaemia. Diab Vasc Dis Res 16(2):153–159. https://doi.org/10.1177/1479164118813890
Bardenheier BH, Elixhauser A, Imperatore G, Devlin HM, Kuklina EV, Geiss LS, Correa A (2013) Variation in prevalence of gestational diabetes mellitus among hospital discharges for obstetric delivery across 23 states in the United States. Diabetes Care 36(5):1209–1214. https://doi.org/10.2337/dc12-0901
Collier A, Abraham EC, Armstrong J, Godwin J, Monteath K, Lindsay R (2017) Reported prevalence of gestational diabetes in Scotland: The relationship with obesity, age, socioeconomic status, smoking and macrosomia, and how many are we missing? J Diabetes Investig 8(2):161–167. https://doi.org/10.1111/jdi.12552
Du M, Liu J, Na H, Zhao Z, Luo S, Wang H (2020) Association between sleep duration in early pregnancy and risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Metab. https://doi.org/10.1016/j.diabet.2020.101217
Momeni Javid F, Simbar M, Dolatian M, Alavi Majd H (2014) Comparison of lifestyles of women with gestational diabetes and healthy pregnant women. Glob J Health Sci 7(2):162–169. https://doi.org/10.5539/gjhs.v7n2p162
Wu L, Han L, Zhan Y, Cui L, Chen W, Ma L, Lv J, Pan R, Zhao D, Xiao Z (2018) Prevalence of gestational diabetes mellitus and associated risk factors in pregnant Chinese women: a cross-sectional study in Huangdao, Qingdao, China. Asia Pac J Clin Nutr 27(2):383–388. https://doi.org/10.6133/apjcn.032017.03
Acknowledgements
We thank the continuous support from the Pedagogical Reform Program of Soochow University. Our special appreciation goes to Shanghai Jiayin Biotechnology Co., Ltd for the expert advice on statistical analysis.
Funding
This study was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions; the special fund of Soochow University for medical college students’ extracurricular scientific research; and the Undergraduate Training Program for Innovation and Entrepreneurship [grant number: 201910285051Z, 202010285043Z, and 202110285150Y].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YC, ML, JN, JL, and YL. The first draft of the manuscript was written by JZ and XY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Consent for publication
All the authors have approved the final manuscript and consented for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Lu, M., Nie, J. et al. Increasing prevalence of gestational diabetes mellitus when carrying the T variant allele of the MTHFR gene C677T polymorphism: a systematic review and meta-analysis. Arch Gynecol Obstet 305, 1193–1202 (2022). https://doi.org/10.1007/s00404-021-06303-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06303-4