Abstract
Purpose
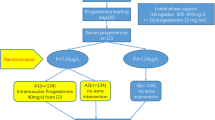
In this study, we intend to evaluate pregnancy outcomes in women who undergo artificial frozen embryo transfer (FET) and stop estradiol (E2) after vaginal ultrasound observation of a gestational sac and heartbeat.
Methods
In this randomized phase III clinical trial, we recruited 291 patients who underwent FET. We randomly assigned 64 pregnant women to a study or a control group after observation of a gestational sac and heartbeat at 6-week gestational age. E2 administration continued until week 12 of gestational age for the control group, but was discontinued for the study group. Progesterone-in-oil administration continued until week 12 of gestational age for both groups. Serum levels for E2 and progesterone were measured on the initial progesterone and embryo transfer (ET) days, and at weeks 6 and 12 of pregnancy in both groups.
Results
The miscarriage rate was 1/32 (3.13%) in the study group and 6/32 (18.75%) in the control group after the intervention and confirmation of a fetal heartbeat. This difference was statistically significant. All patients who remained under intervention, which included 29 in the study group and 24 in the control group, had live births. Although the mean serum E2 and progesterone levels steadily increased from the initial day of progesterone administration to week 12 of gestational age, they were not significantly different between the two groups. Maternal complications were significantly more common in the control group.
Conclusion
Earlier discontinuation of E2 for luteal phase support of FET cycles may be taken into consideration. Additional clinical studies should be conducted to determine an accurate estimation of the time when E2 should be discontinued during FET luteal phase support.
Trial registration
NCT04013438, registered 9 July 2019—Retrospectively registered, https://www.clinicaltrials.gov/ct2/show/NCT04013438?cond=NCT04013438&draw=2&rank=1.



Similar content being viewed by others
References
Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, de Mouzon J, Castilla JA, Erb K, Korsak V, Nyboe Andersen A, IVF-monitoring E (2013) Assisted reproductive technology in Europe, 2009: results generated from European registers by ESHRE. Hum Reprod 28(9):2318–2331
Nekoo EA, Chamani M, Tehrani ES, Rashidi BH, Tanha FD, Kalantari V (2015) Artificial endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with depot gonadotropin releasing hormone agonist in women with regular menses. J Family Reprod Health 9(1):1
Ghobara T, Gelbaya TA, Ayeleke RO (2017) Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003414.pub3
Niu Z, Feng Y, Sun Y, Zhang A, Zhang H (2008) Estrogen level monitoring in artificial frozen-thawed embryo transfer cycles using step-up regime without pituitary suppression: is it necessary? J Exp Clin Assist Reprod 5(1):4
Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ (2017) What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update 23(2):255–261
Groll JM, Usadi RS, Lessey BA, Lininger R, Young SL, Fritz MA (2009) Effects of variations in serum estradiol concentrations on secretory endometrial development and function in experimentally induced cycles in normal women. Fertil Steril 92(6):2058–2061
Trounson A, Mohr L (1983) Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 305(5936):707–709
Jing Z, Xi H, Qianling Z, Lunquan S, Nenghui L, Yanping L (2019) Oestrogen dose tapering during luteal phase does not affect clinical outcomes after hormone replacement treatment–frozen-thawed embryo transfer cycles: a retrospective analysis. Hum Reprod 34(8):1479–1484. https://doi.org/10.1093/humrep/dez096
Liao X, Li Z, Dong X, Zhang H (2014) Comparison between oral and vaginal estrogen usage in inadequate endometrial patients for frozen-thawed blastocysts transfer. Int J Clin Exp Pathol 7(10):6992
Scott R, Navot D, Liu H-C, Rosenwaks Z (1991) A human in vivo model for the luteoplacental shift. Fertil Steril 56(3):481–484
Liu X-R, Mu H-Q, Shi Q, Xiao X-Q, Qi H-B (2012) The optimal duration of progesterone supplementation in pregnant women after IVF/ICSI: a meta-analysis. Reprod Biol Endocrinol 10(1):107
Tong X-M, Zhu H-Y, Zhou F, Huang Q-X, Jiang L-Y, Li C, Lin X-N, Zhang S-Y (2011) Maintenance of early pregnancy without early hormone support after frozen-thawed embryo transfer in hormone replacement treatment cycles. Fertil Steril 95(6):2125 e2115–212 e52117
Balaban B, Brison D, Calderon G, Catt J, Conaghan J, Cowan L, Ebner T, Gardner D, Hardarson T, Lundin K (2011) Alpha scientists in reproductive medicine and ESHRE special interest group of embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 26(6):1270–1283
Papageorgiou T, Guibert J, Goffinet F, Patrat C, Fulla Y, Janssens Y, Zorn J-R (2002) Percentile curves of serum estradiol levels during controlled ovarian stimulation in 905 cycles stimulated with recombinant FSH show that high estradiol is not detrimental to IVF outcome. Hum Reprod 17(11):2846–2850
Paulson RJ (2011) Hormonal induction of endometrial receptivity. Fertil Steril 96(3):530–535
Mirkin S, Nikas G, Hsiu J-G, Díaz J, Oehninger S (2004) Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab 89(11):5742–5752
Gruber I, Just A, Birner M, Lösch A (2007) Serum estradiol/progesterone ratio on day of embryo transfer may predict reproductive outcome following controlled ovarian hyperstimulation and in vitro fertilization. J Exp Clin Assist Reprod 4(1):1
Kalma Y, Granot I, Gnainsky Y, Or Y, Czernobilsky B, Dekel N, Barash A (2009) Endometrial biopsy-induced gene modulation: first evidence for the expression of bladder-transmembranal uroplakin Ib in human endometrium. Fertil Steril 91(4):1042-1049 e1049
Basnayake SK, Volovsky M, Rombauts L, Osianlis T, Vollenhoven B, Healey M (2018) Progesterone concentrations and dosage with frozen embryo transfers–Wha’s best? Aust N Z J Obstet Gynaecol 58(5):533–538
De Ziegler D, Bergeron C, Cornel C, Medalie D, Massai M, Milgrom E, Frydman R, Bouchard P (1992) Effects of luteal estradiol on the secretory transformation of human endometrium and plasma gonadotropins. J Clin Endocrinol Metab 74(2):322–331
Younis JS, Ezra Y, Sherman Y, Simon A, Schenker JG, Laufer N (1994) The effect of estradiol depletion during the luteal phase on endometrial development. Fertil Steril 62(1):103–107
Grossi E, Parisi F, Duca P, Savasi V (2019) Maternal estradiol and progesterone concentrations among singleton spontaneous pregnancies during the first trimester. J Endocrinol Invest. https://doi.org/10.1007/s40618-018-0961-6
Scheffer JB, Scheffer BB, de Souza Aguiar AP, Franca JB, Lozano DM, Fanchin R (2021) A comparison of the effects of three different estrogen used for endometrium preparation on the outcome of day 5 frozen embryo transfer cycle. JBRA Assist Reprod 25(1):104
Behnam-Rasouli M, Nikravesh MR (1997) The abortional and the teratogenical effects of 17-beta estradiol valerate administration during embryonic development in rats. Iran Biomed J 1(4):53–57
Devroey P, Camus M, Palermo G, Smitz J, Van Waesberghe L, Wisanto A, Wijbo I, Van Steirteghem AC (1990) Placental production of estradiol and progesterone after oocyte donation in patients with primary ovarian failure. Am J Obstet Gynecol 162(1):66–70
Carolan M, Wright RJ (2017) Miscarriage at advanced maternal age and the search for meaning. Death Stud 41(3):144–153
Magnus MC, Wilcox AJ, Morken N-H, Weinberg CR, Håberg SE (2019) Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ 364:l869
Hilakivi-Clarke L, de Assis S, Warri A (2013) Exposures to synthetic estrogens at different times during the life, and their effect on breast cancer risk. J Mammary Gland Biol Neoplasia 18(1):25–42
Järvelä IY, Pelkonen S, Uimari O, Mäkikallio K, Puukka K, Ruokonen A, Tekay A, Martikainen H (2014) Controlled ovarian hyperstimulation leads to high progesterone and estradiol levels during early pregnancy. Hum Reprod 29(11):2393–2401
Bagot C, Leishman E, Onyiaodike C, Jordan F, Gibson V, Freeman D (2019) Changes in laboratory markers of thrombotic risk early in the first trimester of pregnancy may be linked to an increase in estradiol and progesterone. Thromb Res 178:47–53
Vinogradova Y, Coupland C, Hippisley-Cox J (2019) Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. https://doi.org/10.1136/bmj.k4810
Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK (2012) Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril 97(6):1374–1379
Royster GD IV, Krishnamoorthy K, Csokmay JM, Yauger BJ, Chason RJ, DeCherney AH, Wolff EF, Hill MJ (2016) Are intracytoplasmic sperm injection and high serum estradiol compounding risk factors for adverse obstetric outcomes in assisted reproductive technology? Fertil Steril 106(2):363-370 e363
Liu J, Zheng J, Lei Y-l, Wen X-f (2019) Effects of endometrial preparations and transferred embryo types on pregnancy outcome from patients with advanced maternal age. Syst Biol Reprod Med 65(2):181–186
Acknowledgements
We express our appreciation to all of the participants in this clinical trial.
Funding
This study was funded by a grant from the Reproductive Biomedicine Research Center, Royan Institute, Tehran, Iran (94000295).
Author information
Authors and Affiliations
Contributions
FG study conception, design, administrative support, and literature review. ZC data collection and assembly, and drafted the manuscript. SV conducted statistical analysis of the data. All the authors were involved in revising the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Ethical Committee of Royan Institute. All of the recruited patients signed an informed consent form for study participation.
Informed consent
All of the recruited patients gave their informed consent to participate in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghaffari, F., Chekini, Z. & Vesali, S. Duration of estradiol supplementation in luteal phase support for frozen embryo transfer in hormone replacement treatment cycles: a randomized, controlled phase III trial. Arch Gynecol Obstet 305, 767–775 (2022). https://doi.org/10.1007/s00404-021-06173-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06173-w