Abstract
Objectives
Ovarian cancer is one of the most fatal gynecologic malignities. miR-16-5p, miR-17-5p, and miR-638 genes were found to have been associated with ovarian cancer in accordance with the data obtained from the previous microarray research performed by Tuncer et al. (J Ovarian Res 13(1):99, 2020). The expression levels of these miRNAs in the peripheral blood samples of 142 ovarian cancer patients, and 97 healthy controls were investigated for performing the validation, and to identify whether these genes were the possible biomarkers to be used in the early diagnosis of high-risk ovarian cancer patients, and in the prognosis of patients.
Methods
The miRNA expression analysis was performed using the miRNA-specific cDNA synthesis, and real-time PCR methods following the RNA isolation from the peripheral blood lymphocytes.
Results
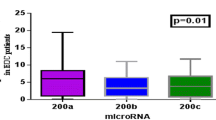
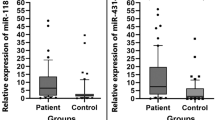
miR-16-5p, miR-17-5p, and miR-638 miRNA gene expression levels were found to have twofold higher expression levels in patient groups compared with the gene expression levels in healthy controls, and were statistically significant (p < 0.05). In addition, the comparison of the miRNA expression levels with the clinical data of patients showed that there was a significant difference with smoking history and the increased expression level of miR-17-5 (p: 0.007). There was a significant difference between the increased expression level of miR-638 with the locally advanced stage, and abdominal/pelvic metastatic patients (p: 0.03).
Conclusions
The obtained data suggest that miR-16-5p, miR-17-5p, and miR-638 molecules might be the noninvasive biomarkers in identifying the ovarian cancer. However, the investigation and monitoring of the changeability of these biomarkers in benign ovarian diseases, and during the treatment must be performed in future studies for identifying the accurate diagnostic, and prognostic features of miRNAs.


Similar content being viewed by others
Availability of the data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article.
References
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Dong X et al (2014) Advances in tumor markers of ovarian cancer for early diagnosis. Indian J Cancer 51(Suppl 3):e72–e76
Bottoni P, Scatena R (2015) The role of CA 125 as tumor marker: biochemical and clinical aspects. Adv Exp Med Biol 867:229–244
Moore LE et al (2012) Proteomic biomarkers in combination with CA 125 for detection of epithelial ovarian cancer using prediagnostic serum samples from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Cancer 118(1):91–100
Elias KM, Guo J, Bast RC Jr (2018) Early detection of ovarian cancer. Hematol Oncol Clin N Am 32(6):903–914
Mahdian-Shakib A et al (2016) Differential role of microRNAs in prognosis, diagnosis, and therapy of ovarian cancer. Biomed Pharmacother 84:592–600
Boyd J, Rubin SC (1997) Hereditary ovarian cancer: molecular genetics and clinical implications. Gynecol Oncol 64(2):196–206
Webb PM, Jordan SJ (2017) Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 41:3–14
Salehi F et al (2008) Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. J Toxicol Environ Health B Crit Rev 11(3–4):301–321
Kurman RJ, Shih-Ie M (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34(3):433–443
Kim J et al (2018) Cell origins of high-grade serous ovarian cancer. Cancers (Basel) 10(11):433
Kurman RJ, Shih-Ie M (2016) The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol 186(4):733–747
Katz B et al (2015) MicroRNAs in ovarian cancer. Hum Pathol 46(9):1245–1256
Peng Y, Croce CM (2016) The role of MicroRNAs in human cancer. Signal Transduct Target Ther 1:15004
Tsuchiya S, Okuno Y, Tsujimoto G (2006) MicroRNA: biogenetic and functional mechanisms and involvements in cell differentiation and cancer. J Pharmacol Sci 101(4):267–270
Ahmed FE (2007) Role of miRNA in carcinogenesis and biomarker selection: a methodological view. Expert Rev Mol Diagn 7(5):569–603
Zheng D (2017) Non-coding RNAs and ovarian diseases. Mol Med Rep 1435–1440 (Review)
Lei L et al (2010) The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 315(1–2):63–73
Tuncer SB et al (2019) The expression levels of miRNA-15a and miRNA-16-1 in circulating tumor cells of patients with diffuse large B cell lymphoma. Mol Biol Rep 46(1):975–980
Erdogan OS et al (2020) Genome-wide methylation profiles in monozygotic twins with discordance for ovarian carcinoma. Oncol Lett 20(6):357
Shenouda SK, Alahari SK (2009) MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev 28(3–4):369–378
Costa PM, Pedroso-de-Lima MC (2013) MicroRNAs as molecular targets for cancer therapy: on the modulation of microRNA expression. Pharmaceuticals (Basel) 6(10):1195–1220
Erson AE, Petty EM (2009) miRNAs and cancer: new research developments and potential clinical applications. Cancer Biol Ther 8(24):2317–2322
Cowland JB, Hother C, Gronbaek K (2007) MicroRNAs and cancer. APMIS 115(10):1090–1106
Lu J et al (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838
Zhang J et al (2015) Circulating MiR-16-5p and MiR-19b-3p as two novel potential biomarkers to indicate progression of gastric cancer. Theranostics 5(7):733–745
Xing Z et al (2014) MicroRNAs and anticancer drugs. Acta Biochim Biophys Sin (Shanghai) 46(3):233–239
Tuncer SB et al (2020) miRNA expression profile changes in the peripheral blood of monozygotic discordant twins for epithelial ovarian carcinoma: potential new biomarkers for early diagnosis and prognosis of ovarian carcinoma. J Ovarian Res 13(1):99
Chen SN et al (2019) MicroRNA in ovarian cancer: biology, pathogenesis, and therapeutic opportunities. Int J Environ Res Public Health 16(9):1510
Wang X et al (2019) Circulating microRNAs as novel potential diagnostic biomarkers for ovarian cancer: a systematic review and updated meta-analysis. J Ovarian Res 12(1):24
Pal MK et al (2015) MicroRNA: a new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol Med 12(4):328–341
Lengyel E (2010) Ovarian cancer development and metastasis. Am J Pathol 177(3):1053–1064
Calin GA et al (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 101(9):2999–3004
Jay C et al (2007) miRNA profiling for diagnosis and prognosis of human cancer. DNA Cell Biol 26(5):293–300
Zhang B, Cai FF, Zhong XY (2011) An overview of biomarkers for the ovarian cancer diagnosis. Eur J Obstet Gynecol Reprod Biol 158(2):119–123
McGinnis LK, Luense LJ, Christenson LK (2015) MicroRNA in ovarian biology and disease. Cold Spring Harb Perspect Med 5(9):a022962
Montazerian M, Yasari F, Aghaalikhani N (2018) Ovarian extracellular MicroRNAs as the potential non-invasive biomarkers: an update. Biomed Pharmacother 106:1633–1640
Donadeu FX, Schauer SN (2013) Differential miRNA expression between equine ovulatory and anovulatory follicles. Domest Anim Endocrinol 45(3):122–125
Imbar T, Eisenberg I (2014) Regulatory role of microRNAs in ovarian function. Fertil Steril 101(6):1524–1530
Yerushalmi GM et al (2018) Characterization of the miRNA regulators of the human ovulatory cascade. Sci Rep 8(1):15605
Machtinger R et al (2017) Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: an exploratory study. J Assist Reprod Genet 34(4):525–533
Wang F et al (2009) Down-regulation of the cyclin E1 oncogene expression by microRNA-16-1 induces cell cycle arrest in human cancer cells. BMB Rep 42(11):725–730
Zhang X et al (2018) Biology of MiR-17-92 cluster and its progress in lung cancer. Int J Med Sci 15(13):1443–1448
Volinia S et al (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103(7):2257–2261
Chen Q et al (2013) Prognostic significance of serum miR-17-5p in lung cancer. Med Oncol 30(1):353
Qu Y et al (2016) MiR-17-5p regulates cell proliferation and migration by targeting transforming growth factor-beta receptor 2 in gastric cancer. Oncotarget 7(22):33286–33296
Zhu Y et al (2018) MiR-17-5p enhances pancreatic cancer proliferation by altering cell cycle profiles via disruption of RBL2/E2F4-repressing complexes. Cancer Lett 412:59–68
Bhattacharya A et al (2015) miR-638 promotes melanoma metastasis and protects melanoma cells from apoptosis and autophagy. Oncotarget 6(5):2966–2980
Funding
The present work was supported by the Research Fund of Istanbul University. Project No: TDK-2018-29694.
Author information
Authors and Affiliations
Contributions
ASM project development, data collection, manuscript writing. TSB study concept, and design, data collection. AOD, SEO, KES, SP data collection. OS statistical analysis. YH project development, data collection, check of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare (Mukaddes Avsar Saral, Seref Bugra Tuncer, Demet Akdeniz Odemis, Ozge Sukruoglu Erdogan, Seda Kilic Erciyas, Pınar Saip, Sevda Ozel, Hulya Yazici).
Ethics approval
All the performed human participant involving procedures were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Istanbul Faculty of Medicine in Istanbul University (Ethics Approval No: 2018/190).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding the publishing of their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saral, M.A., Tuncer, S.B., Odemis, D.A. et al. New biomarkers in peripheral blood of patients with ovarian cancer: high expression levels of miR-16-5p, miR-17-5p, and miR-638. Arch Gynecol Obstet 305, 193–201 (2022). https://doi.org/10.1007/s00404-021-06138-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06138-z