Abstract
Purpose
To investigate the efficacy and safety of Nr-CWS on treatment of cervical HSIL.
Method
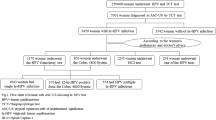
In this observational study, 16 patients were treated with Nr-CWS 1 time every 2 days for 6 times as one-course group (OC group), the other 184 patients were treated with Nr-CWS 1 time every 2 days for 12 times between 2 menstruations as two-course group (TC group). The medical information including age, HPV assay, vaginal–cervical cytology, and the pathological result of biopsy before and after treatment was collected. All patients were followed up at least twice after treatment. The LEEP was performed once the patients with persistent HR-HPV infection and/or abnormal TCT after the second follow-up.
Results
The cytology remission rate of cervical HSIL in OC and TC group was 100.0% and 87.8%, respectively, which were significant higher than the control (25.0%) with the P value of 2.00 × 10–3 and 2.06 × 10–4. Furthermore, HPV clearance rate was 87.5% and 70.2% in OC and TC group, respectively, which were significant higher than control (32.4%) with the P value of 2.74 × 10−4 and 2.18 × 10–5, respectively. Moreover, the more severe of cytology, the worse effect of HPV clearance for the HPV remission was 75.4%, 68.3%, 67.4%, 65.6% and 64.3% in the negative, LSIL, ASC-US, ASC-H, and HSIL group. 12 patients underwent LEEP after Nr-CWS treatment, 9 (75%) had persistent HSIL and 44.4% cases were found HSIL lesion in the cervical canal. There was no serious adverse reaction observed during treatment and follow-up, four patients were pregnant after treatment and no adverse pregnancy outcomes were observed.
Conclusion
Nr-CWS is an effective and safe drug for treatment of cervical HSIL for Chinese women, especially for cases without lesions in cervical canal.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ (2006) HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol 208(2):152–164. https://doi.org/10.1002/path.1866
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S (2007) Human papillomavirus and cervical cancer. Lancet (London, England) 370(9590):890–907. https://doi.org/10.1016/s0140-6736(07)61416-0
Cohen PA, Jhingran A, Oaknin A, Denny L (2019) Cervical cancer. Lancet (London, England) 393(10167):169–182. https://doi.org/10.1016/s0140-6736(18)32470-x
Dijkstra MG, van Zummeren M, Rozendaal L, van Kemenade FJ, Helmerhorst TJ, Snijders PJ, Meijer CJ, Berkhof J (2016) Safety of extending screening intervals beyond five years in cervical screening programmes with testing for high risk human papillomavirus: 14 year follow-up of population based randomised cohort in the Netherlands. BMJ (Clinical research ed) 355:i4924. https://doi.org/10.1136/bmj.i4924
Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, Mayrand MH, Ruiz-Sternberg AM, Stapleton JT, Wiley DJ, Ferenczy A, Kurman R, Ronnett BM, Stoler MH, Cuzick J, Garland SM, Kjaer SK, Bautista OM, Haupt R, Moeller E, Ritter M, Roberts CC, Shields C, Luxembourg A (2017) Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet (London, England) 390(10108):2143–2159. https://doi.org/10.1016/s0140-6736(17)31821-4
Palmer T, Wallace L, Pollock KG, Cuschieri K, Robertson C, Kavanagh K, Cruickshank M (2019) Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12–13 in Scotland: retrospective population study. BMJ (Clinical research ed) 365:l1161. https://doi.org/10.1136/bmj.l1161
Jing L, Dan W, Zhunan L, Ying X, Yi C (2018) Residual lesions in uterine specimens after loop electrosurgical excision procedure in patients with CIN. Arch Gynecol Obstet 298(4):805–812. https://doi.org/10.1007/s00404-018-4881-7
Fujisawa T, Yamaguchi Y (1996) Postoperative immunostimulation after complete resection improves survival of patients with stage I nonsmall cell lung carcinoma. Cancer 78(9):1892–1898. https://doi.org/10.1002/(sici)1097-0142(19961101)78:9%3c1892::aid-cncr8%3e3.3.co;2-u
Zhao J, Du HJ, Liao QP (2007) Effect of Nocardia rubra cell wall skeleton on the growth of HeLa cell line infected with HPV. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 21(1):20–22
Zhao J, Zhan SB, Li XQ, Zhou L, Yang YJ, Liao QP (2007) Effect of Nocardia rubra cell wall skeleton (Nr-CWS) on oncogenicity of TC-1 cells and anti-human papillomavirus effect of Nr-CWS in lower genital tract of women. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 21(4):340–342
Smith JH (2002) Bethesda 2001. Cytopathol Off J Br Soc Clin Cytol 13(1):4–10
Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO classification of tumours of female reproductive organs. International Agency for Research on Cancer (IARC), Lyon
Fu Y, Bao Y, Hui Y, Gao X, Yang M, Chang J (2016) Topical photodynamic therapy with 5-aminolevulinic acid for cervical high-risk HPV infection. Photodiagn Photodyn Ther 13:29–33. https://doi.org/10.1016/j.pdpdt.2015.12.004
Case AS, Rocconi RP, Straughn JM, Wang W, Roark K, Waltman EE, Huh WK (2006) Cervical intraepithelial neoplasia in adolescent women: incidence and treatment outcomes. Obstet Gynecol 108(6):1369–1374. https://doi.org/10.1097/01.aog.0000245448.19446.81
Kucera E, Sliutz G, Czerwenka K, Breitenecker G, Leodolter S, Reinthaller A (2001) Is high-risk human papillomavirus infection associated with cervical intraepithelial neoplasia eliminated after conization by large-loop excision of the transformation zone? Eur J Obstet Gynecol Reprod Biol 100(1):72–76. https://doi.org/10.1016/s0301-2115(01)00457-2
Lörincz AT, Richart RM (2003) Human papillomavirus DNA testing as an adjunct to cytology in cervical screening programs. Arch Pathol Lab Med 127(8):959–968. https://doi.org/10.1043/1543-2165(2003)127%3c959:hpdtaa%3e2.0.co;2
Dong B, Sun P, Ruan G, Huang W, Mao X, Kang Y, Pan D, Lin F (2018) Type-specific high-risk human papillomavirus viral load as a viable triage indicator for high-grade squamous intraepithelial lesion: a nested case–control study. Cancer Manag Res 10:4839–4851. https://doi.org/10.2147/cmar.s179724
Kang WD, Choi HS, Kim SM (2013) Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol 130(2):264–268. https://doi.org/10.1016/j.ygyno.2013.04.050
Chang DY, Song SH, You S, Lee J, Kim J, Racanelli V, Son H, Shin EC (2014) Programmed death-1 (PD-1)-dependent functional impairment of CD4(+) T cells in recurrent genital papilloma. Clin Exp Med 14(3):305–313. https://doi.org/10.1007/s10238-013-0245-6
Wang G, Wu J, Miao M, Dou H, Nan N, Shi M, Yu G, Shan F (2017) Nocardia rubra cell-wall skeleton promotes CD4 T cell activation and drives Th1 immune response. Int J Biol Macromol 101:398–407. https://doi.org/10.1016/j.ijbiomac.2017.03.060
Meng Y, Sun J, Wang X, Ma Y, Kong C, Zhang G, Dou H, Nan N, Shi M, Yu T, Piao H (2019) The biological macromolecule Nocardia rubra cell-wall skeleton as an avenue for cell-based immunotherapy. J Cell Physiol. https://doi.org/10.1002/jcp.28182
Author information
Authors and Affiliations
Contributions
YZ and YC designed experiments. HF and TW carried out experiments. XP analyzed experimental results. JZ wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in the manuscript entitled, “The safety and efficacy of a novel method for treatment of HSIL”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, J., Feng, H., Wang, T. et al. The safety and efficacy of a novel method for treatment of HSIL. Arch Gynecol Obstet 304, 1291–1298 (2021). https://doi.org/10.1007/s00404-021-06047-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06047-1