Abstract
Purpose
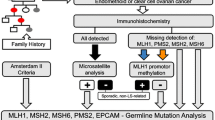
Current guidelines for Lynch syndrome detection in endometrial cancer (EC) patients rely either on risk evaluation, based on personal/family history, or detection of mismatch repair (MMR) deficiency on tumor tissue. We present a combined screening algorithm for Lynch syndrome.
Methods
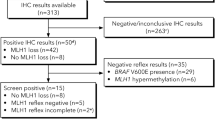
In this study, 213 consecutive patients treated for EC at Kliniken Essen-Mitte between 2014 and 2018 were included. Personal/family history was evaluated by the Amsterdam II, revised Bethesda/German-DKG criteria and prediction model PREMM5. MMR testing was performed by immunohistochemistry (IHC) and/or polymerase chain reaction (PCR) based microsatellite analysis on tumor tissue. MLH1 promoter methylation analysis was performed in case of MLH1 loss or microsatellite instability.
Results
Based on personal/family history 2/213 (Amsterdam II), 31/213 (revised Bethesda/German-DKG) and 149/213 (PREMM5) patients were identified as at risk for Lynch syndrome. MMR analysis was performed by IHC in 51.2%, by PCR in 32.4%, and in 16.4% of patients both methods were used. MMR deficiency was detected in 20.6% (44/213). Methylation analysis was performed in 27 patients of whom, 22 (81.4%) showed MLH1 promoter hypermethylation. Only 9% of MMR deficient patients were identified as at risk for Lynch syndrome by the revised Bethesda/German-DKG criteria. A pathogenic germline mutation was discovered in 3 out of 20 patients that underwent genetic testing. None of these patients were younger than 50 years or had a family history of Lynch syndrome-associated malignancies.
Conclusion
General MMR assessment is a feasible strategy to improve the detection of Lynch Syndrome in patients with EC.



Similar content being viewed by others
References
Koch-Institut R (2017) Krebs in Deutschland für 2013/2014. Robert Koch-Institut. https://doi.org/10.17886/rkipubl-2017-007
Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C, Group E-E-EECCW (2016) ESMO-ESGO-ESTRO Consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer 26(1):2–30. https://doi.org/10.1097/IGC.0000000000000609
Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, La Jeunesse J, Nakagawa H, Westman JA, Prior TW, Clendenning M, Penzone P, Lombardi J, Dunn P, Cohn DE, Copeland L, Eaton L, Fowler J, Lewandowski G, Vaccarello L, Bell J, Reid G, de la Chapelle A (2006) Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 66(15):7810–7817. https://doi.org/10.1158/0008-5472.CAN-06-1114
Buchanan DD, Tan YY, Walsh MD, Clendenning M, Metcalf AM, Ferguson K, Arnold ST, Thompson BA, Lose FA, Parsons MT, Walters RJ, Pearson SA, Cummings M, Oehler MK, Blomfield PB, Quinn MA, Kirk JA, Stewart CJ, Obermair A, Young JP, Webb PM, Spurdle AB (2014) Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol 32(2):90–100. https://doi.org/10.1200/JCO.2013.51.2129
Berends MJ, Wu Y, Sijmons RH, van der Sluis T, Ek WB, Ligtenberg MJ, Arts NJ, ten Hoor KA, Kleibeuker JH, de Vries EG, Mourits MJ, Hollema H, Buys CH, Hofstra RM, van der Zee AG (2003) Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol 21(23):4364–4370. https://doi.org/10.1200/JCO.2003.04.094
Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, White KG, Luthra R, Gershenson DM, Broaddus RR (2007) Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol 25(33):5158–5164. https://doi.org/10.1200/JCO.2007.10.8597
Tafe LJ, Riggs ER, Tsongalis GJ (2013) Lynch syndrome presenting as endometrial cancer. Clin Chem 60(1):111–121. https://doi.org/10.1373/clinchem.2013.206888
Egoavil C, Alenda C, Castillejo A, Paya A, Peiro G, Sanchez-Heras AB, Castillejo MI, Rojas E, Barbera VM, Ciguenza S, Lopez JA, Pinero O, Roman MJ, Martinez-Escoriza JC, Guarinos C, Perez-Carbonell L, Aranda FI, Soto JL (2013) Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. PLoS ONE 8(11):e79737. https://doi.org/10.1371/journal.pone.0079737
Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, Peltomaki P, Mecklin JP, Jarvinen HJ (1999) Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81(2):214–218. https://doi.org/10.1002/(sici)1097-0215(19990412)81:2%3c214::aid-ijc8%3e3.0.co;2-l
Vasen HF, Offerhaus GJ, den Hartog Jager FC, Menko FH, Nagengast FM, Griffioen G, van Hogezand RB, Heintz AP (1990) The tumour spectrum in hereditary non-polyposis colorectal cancer: a study of 24 kindreds in the Netherlands. Int J Cancer 46(1):31–34. https://doi.org/10.1002/ijc.2910460108
Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, Bandipalliam P, Chen LM, Allen B, Conrad P, Terdiman J, Sun C, Daniels M, Burke T, Gershenson DM, Lynch H, Lynch P, Broaddus RR (2005) Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol 105(3):569–574. https://doi.org/10.1097/01.AOG.0000154885.44002.ae
Vasen HF, Watson P, Mecklin JP, Jass JR, Green JS, Nomizu T, Muller H, Lynch HT (1994) The epidemiology of endometrial cancer in hereditary nonpolyposis colorectal cancer. Anticancer Res 14(4B):1675–1678
Koornstra JJ, Mourits MJ, Sijmons RH, Leliveld AM, Hollema H, Kleibeuker JH (2009) Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol 10(4):400–408. https://doi.org/10.1016/S1470-2045(09)70041-5
Pinol V, Castells A, Andreu M, Castellvi-Bel S, Alenda C, Llor X, Xicola RM, Rodriguez-Moranta F, Paya A, Jover R, Bessa X, Gastrointestinal Oncology Group of the Spanish Gastroenterological A (2005) Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA 293(16):1986–1994. https://doi.org/10.1001/jama.293.16.1986
Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A (2005) Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 352(18):1851–1860. https://doi.org/10.1056/NEJMoa043146
Kastrinos F, Uno H, Ukaegbu C, Alvero C, McFarland A, Yurgelun MB, Kulke MH, Schrag D, Meyerhardt JA, Fuchs CS, Mayer RJ, Ng K, Steyerberg EW, Syngal S (2017) Development and validation of the PREMM5 model for comprehensive risk assessment of Lynch syndrome. J Clin Oncol 35(19):2165–2172. https://doi.org/10.1200/JCO.2016.69.6120
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF): Diagnostik, Therapie und Nachsorge der Patientinnen mit Endometriumkarzinom, Langversion 1.0, 2018, AWMF Registernummer: 032/034-OL, http://www.leitlinienprogramm-onkologie.de/leitlinien/endometriumkarzinom/ (abgerufen am: 12.10.2019).
Wui-Jin K, Nadeem RA-R, Sarah B, Kristin B, Susana MC, Kathleen RC, Hye Sook C, Christina C, David C, Marta Ann C, Shari D, Oliver D, Patricia JE, Christine MF, Peter F, David KG, Suzanne G, Ernest H, Susan H, Warner KH, John RL, Andrea M, David M, Christa N, Larissa N, Amanda Nickles F, Steven WR, Reynolds RK, Todd T, Stefanie U, Emily W, Catheryn MY, Nicole RM, Jillian LS (2018) Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16(2):170–199. https://doi.org/10.6004/jnccn.2018.0006
Randall LM, Pothuri B, Swisher EM, Diaz JP, Buchanan A, Witkop CT, Bethan Powell C, Smith EB, Robson ME, Boyd J, Coleman RL, Lu K (2017) Multi-disciplinary summit on genetics services for women with gynecologic cancers: a society of gynecologic oncology white paper. Gynecol Oncol 146(2):217–224. https://doi.org/10.1016/j.ygyno.2017.06.002
Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti A, Fotopoulou C, Gonzalez Martin A, Lax S, Lorusso D, Marth C, Morice P, Nout RA, O’Donnell D, Querleu D, Raspollini MR, Sehouli J, Sturdza A, Taylor A, Westermann A, Wimberger P, Colombo N, Planchamp F, Creutzberg CL (2021) ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 31(1):12–39. https://doi.org/10.1136/ijgc-2020-002230
Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, Ramirez N, Pritchard CC, Hampel H, Chassen AS, Simmons LV, Schmidt AP, Gao F, Brinton LA, Backes F, Landrum LM, Geller MA, DiSilvestro PA, Pearl ML, Lele SB, Powell MA, Zaino RJ, Mutch D (2015) Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG oncology and gynecologic oncology group study. J Clin Oncol 33(36):4301–4308. https://doi.org/10.1200/JCO.2015.63.9518
Kahn RM, Gordhandas S, Maddy BP, Baltich Nelson B, Askin G, Christos PJ, Caputo TA, Chapman-Davis E, Holcomb K, Frey MK (2019) Universal endometrial cancer tumor typing: How much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer 125(18):3172–3183. https://doi.org/10.1002/cncr.32203
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268. https://doi.org/10.1093/jnci/djh034
Vasen HF, Watson P, Mecklin JP, Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116(6):1453–1456. https://doi.org/10.1016/s0016-5085(99)70510-x
Nardon E, Glavac D, Benhattar J, Groenen PJ, Hofler G, Hofler H, Jung A, Keller G, Kirchner T, Lessi F, Ligtenberg MJ, Mazzanti CM, Winter G, Stanta G (2010) A multicenter study to validate the reproducibility of MSI testing with a panel of 5 quasimonomorphic mononucleotide repeats. Diagn Mol Pathol 19(4):236–242. https://doi.org/10.1097/PDM.0b013e3181db67af
Bettstetter M, Dechant S, Ruemmele P, Vogel C, Kurz K, Morak M, Keller G, Holinski-Feder E, Hofstaedter F, Dietmaier W (2008) MethyQESD, a robust and fast method for quantitative methylation analyses in HNPCC diagnostics using formalin-fixed and paraffin-embedded tissue samples. Lab Invest 88(12):1367–1375. https://doi.org/10.1038/labinvest.2008.100
Heitz F, Amant F, Fotopoulou C, Battista MJ, Wimberger P, Traut A, Fisseler-Eckhoff A, Harter P, Vandenput I, Sehouli J, Schmidt M, Kimmig R, du Bois R, du Bois A (2014) Synchronous ovarian and endometrial cancer–an international multicenter case-control study. Int J Gynecol Cancer 24(1):54–60. https://doi.org/10.1097/IGC.0000000000000019
Lu KH, Ring KL (2015) One size may not fit all: the debate of universal tumor testing for Lynch syndrome. Gynecol Oncol 137(1):2–3. https://doi.org/10.1016/j.ygyno.2015.03.011
Lin DI, Hecht JL (2016) Targeted screening with combined age- and morphology-based criteria enriches detection of Lynch syndrome in endometrial cancer. Int J Surg Pathol 24(4):297–305. https://doi.org/10.1177/1066896916629782
Dillon JL, Gonzalez JL, DeMars L, Bloch KJ, Tafe LJ (2017) Universal screening for Lynch syndrome in endometrial cancers: frequency of germline mutations and identification of patients with Lynch-like syndrome. Hum Pathol 70:121–128. https://doi.org/10.1016/j.humpath.2017.10.022
Kast K, Dobberschutz C, Sadowski CE, Pistorius S, Wimberger P (2016) Prevalence of Lynch syndrome in unselected patients with endometrial or ovarian cancer. Arch Gynecol Obstet 294(6):1299–1303. https://doi.org/10.1007/s00404-016-4180-0
Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348(10):919–932. https://doi.org/10.1056/NEJMra012242
Mills AM, Liou S, Ford JM, Berek JS, Pai RK, Longacre TA (2014) Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol 38(11):1501–1509. https://doi.org/10.1097/pas.0000000000000321
Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, Lynch P, Burke W, Press N (2006) Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 296(12):1507–1517. https://doi.org/10.1001/jama.296.12.1507
Singh H, Schiesser R, Anand G, Richardson PA, El-Serag HB (2010) Underdiagnosis of Lynch syndrome involves more than family history criteria. Clin Gastroenterol Hepatol 8(6):523–529. https://doi.org/10.1016/j.cgh.2010.03.010
Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Hartman AR, Daniels MS, Broaddus RR (2016) Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol 29(11):1381–1389. https://doi.org/10.1038/modpathol.2016.135
Rubio I, Ibanez-Feijoo E, Andres L, Aguirre E, Balmana J, Blay P, Llort G, Gonzalez-Santiago S, Maortua H, Tejada MI, Martinez-Bouzas C (2016) Analysis of Lynch syndrome mismatch repair genes in women with endometrial cancer. Oncology 91(3):171–176. https://doi.org/10.1159/000447972
Trano G, Wasmuth HH, Sjursen W, Hofsli E, Vatten LJ (2009) Awareness of heredity in colorectal cancer patients is insufficient among clinicians: a Norwegian population-based study. Colorectal Dis 11(5):456–461. https://doi.org/10.1111/j.1463-1318.2009.01830.x
Sjursen W, Haukanes BI, Grindedal EM, Aarset H, Stormorken A, Engebretsen LF, Jonsrud C, Bjornevoll I, Andresen PA, Ariansen S, Lavik LA, Gilde B, Bowitz-Lothe IM, Maehle L, Moller P (2010) Current clinical criteria for Lynch syndrome are not sensitive enough to identify MSH6 mutation carriers. J Med Genet 47(9):579–585. https://doi.org/10.1136/jmg.2010.077677
Shia J (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn 10(4):293–300. https://doi.org/10.2353/jmoldx.2008.080031
Bharati R, Jenkins MA, Lindor NM, Le Marchand L, Gallinger S, Haile RW, Newcomb PA, Hopper JL, Win AK (2014) Does risk of endometrial cancer for women without a germline mutation in a DNA mismatch repair gene depend on family history of endometrial cancer or colorectal cancer? Gynecol Oncol 133(2):287–292. https://doi.org/10.1016/j.ygyno.2014.03.011
Bruegl AS, Ring KL, Daniels M, Fellman BM, Urbauer DL, Broaddus RR (2017) Clinical challenges associated with universal screening for Lynch syndrome-associated endometrial cancer. Cancer Prev Res (Philadelphia, Pa) 10(2):108–115. https://doi.org/10.1158/1940-6207.Capr-16-0219
Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497(7447):67–73. https://doi.org/10.1038/nature12113
https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (2017). Accessed 19 Sept 2020
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Study conception and design: BA, NP, AdB, PH. Material preparation: SH, BSM, RS, KR, BA, NP. Data collection and analysis: BA, NP, AdB, PH, AT. Manuscript writing: BA, NP, TB. Manuscript editing and final approval: all authors.
Corresponding author
Ethics declarations
Conflict of interest
NP: none. AdB: reports personal fees from Roche, Astra Zeneca, Tesaro, Clovis, Pfizer, Genmab, Pharmar, Biocad, and MSD, outside the submitted work. PH: reports grants and personal fees from Astra Zeneca, Roche, Tesaro, and Public funding (ASCO, DKH, DFG), personal fees from Sotio, Stryker, Zai Lab, MSD, Clovis, and Immunogen, grants from GSK, Boehringer Ingelheim, Medac, and Genmab, outside the submitted work. SP: reports non-financial support from Tesaro, outside the submitted work. RS: reports personal fees from Astra Zeneca, Tesaro, Clovis, outside the submitted work. KR: reports personal fees from Astra Zeneca, Tesaro, Roche, non-financial support from Tesaro, outside the submitted work. BSM: none. JS: none. SH: none.FH: reports personal fees from Roche, AstraZeneca, and Clovis, personal fees and non-financial support from PharmaMar and Tesaro, outside the submitted work. StS: reports personal fees from Astra Zeneca, Tesaro outside the submitted work. TB: reports personal fees from GSK/Tesaro, grants from Amgen and non-financial support from Roche and Amgen outside of the submitted work. AT: reports no financial support outside the submitted work. SE: reports non-financial support from Tesaro, outside the submitted work. BA: reports personal fees and non-financial support from Roche and Tesaro, personal fees from Amgen, AstraZeneca, Clovis and Celgene, non-financial support from PharmaMar, outside the submitted work.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The Ethikkommission Ärztekammer Nordrhein stated that retrospective analyses and the publication of fully anonymised data are coverd by their statute (approval 51-2017).Independently, all analyses were carried out in accordance with the provisions of the German Genetic Diagnostics Act after extensive counsleling and written consent of the affected person.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
404_2021_6006_MOESM1_ESM.pptx
Supplementary file1 Figure 1: Molecular characteristic / mismatch repair result based on test method; MMR mismatch repair status; N number of patients; IHC immunohistochemistry; MS microsatellite; PCR polymerase chain reaction (PPTX 41 KB) (PPTX 41 KB)
Rights and permissions
About this article
Cite this article
Pauly, N., Baert, T., Schmutzler, R. et al. Modern day screening for Lynch syndrome in endometrial cancer: the KEM experience. Arch Gynecol Obstet 304, 975–984 (2021). https://doi.org/10.1007/s00404-021-06006-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06006-w