Abstract
Purpose
“Real-world” data incorporates studies performed outside of controlled environments, allowing for a better understanding of the effects of treatment in routine clinical practice. We, therefore, performed a systematic review to summarise available “real-world studies” reporting on the use of ulipristal acetate (UPA) for management of uterine fibroids.
Methods
We designed a prospective protocol according to PRISMA guidelines and registered it with PROSPERO (ID: CRD42019151393). We searched all major databases for relevant citations until 20th September 2019. Our screen included studies for risk of bias using an adapted structured quality assessment tool. Random-effects meta-analysis was used to calculate proportion estimates for each outcome including 95% confidence interval. Reported heterogeneity was assessed using I2.
Results
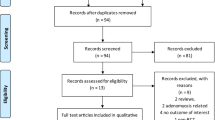
Initial search yielded 755 studies and 13 were included in the final synthesis. Administration of UPA resulted in reduction in the size of fibroids in 56.5% of women, improved menorrhagia in 83% of women, improved perception of pain in 80.1% of women and lead to an improvement in global symptom scores in 85.2% of women. Mean reduction in surgical blood loss and surgical time with use of UPA was 59.85 ml and 12.47 min, respectively. Qualitative analysis suggested that there was no difference in overall surgical experience for patients treated with UPA compared to those without pre-treatment.
Conclusions
Our findings are consistent with previously reported data that UPA is an acceptable management option for women with fibroids. However, it provides limited benefits when used as a pre-operative adjunct, in terms of blood loss and surgical time.




Similar content being viewed by others
Data availability
Available on request.
References
Cramer SF, Patel A (1990) The frequency of uterine leiomyomas. Am J Clin Pathol 94(4):435–438. https://doi.org/10.1093/ajcp/94.4.435
Parker WH (2007) Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril 87(4):725–736. https://doi.org/10.1016/j.fertnstert.2007.01.093
Lumsden MA, Hamoodi I, Gupta J, Hickey M (2015) Fibroids: diagnosis and management. BMJ 351:h4887. https://doi.org/10.1136/bmj.h4887 (Published 2015 Oct 13)
Marshall LM, Spiegelman D, Barbieri RL et al (1997) Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 90(6):967–973
Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH (2012) The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 206(3):211.e1-211.e2119. https://doi.org/10.1016/j.ajog.2011.12.002
Lewis TD, Malik M, Britten J, San Pablo AM, Catherino WH (2018) A comprehensive review of the pharmacologic management of uterine leiomyoma. Biomed Res Int. 2018:2414609. https://doi.org/10.1155/2018/2414609 (Published 2018 Jan 28)
Rabe T, Saenger N, Ebert AD et al (2018) Selective progesterone receptor modulators for the medical treatment of uterine fibroids with a focus on ulipristal acetate. Biomed Res Int. 2018:1374821. https://doi.org/10.1155/2018/1374821 (Published 2018 Jun 24 [published correction appears in Biomed Res Int. 2018 Nov 28;2018:6124628])
Ghonim M, Magdy R, Sabbour M, Ghonim M, Nabhan A (2019) A systematic review and meta-analysis of ulipristal acetate for symptomatic uterine fibroids. Int J Gynaecol Obstet 146(2):141–148. https://doi.org/10.1002/ijgo.12868
Donnez J, Tomaszewski J, Vázquez F et al (2012) Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 366(5):421–432. https://doi.org/10.1056/NEJMoa1103180
Donnez J, Tatarchuk TF, Bouchard P et al (2012) Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 366(5):409–420. https://doi.org/10.1056/NEJMoa1103182
Simon J, Catherino W, Segars J, Blakesley R, Chan A, Sniukiene V, Al-Hendy A (2016) First US-based phase 3 study of ulipristal acetate (UPA) for symptomatic uterine fibroids (UF): results of VENUS-I. Fertil Steril 106(3):e376. https://doi.org/10.1016/j.fertnstert.2016.08.005
Liu J, Soper D, Lukes A, Gee P, Kimble T, Kroll R et al (2017) VENUS II: the second us-based phase 3 study of ulipristal acetate (UPA) for treatment of symptomatic uterine fibroids (UF). Fertil Steril 108(3):e27–e28. https://doi.org/10.1016/j.fertnstert.2017.07.096
Makady A, de Boer A, Hillege H, Klungel O, Goettsch W, (on behalf of GetReal Work Package 1) (2017) What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health. 20(7):858–865. https://doi.org/10.1016/j.jval.2017.03.008
Eichler HG, Abadie E, Breckenridge A et al (2011) Bridging the efficacy-effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nat Rev Drug Discov 10(7):495–506. https://doi.org/10.1038/nrd3501 (Published 2011 Jul 1)
Increasing use of health and social care data in guidance development. (2019). Retrieved from https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-guidelines/how-we-develop-nice-guidelines/data-and-analytics-statement-of-intent. Accessed 26 Apr 2020
Association of The British Pharmaceutical Industry. 2011. Demonstrating Value with Real World data. Retrieved from: https://www.abpi.org.uk/publications/real-world-data. Accessed 23 May 2020
Odejinmi F, Oliver R, Mallick R (2017) Is ulipristal acetate the new drug of choice for the medical management of uterine fibroids? Res ipsa loquitur? Womens Health (Lond) 13(3):98–105. https://doi.org/10.1177/1745505717740218
Liu JH, Soper D, Lukes A et al (2018) Ulipristal acetate for treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol 132(5):1241–1251. https://doi.org/10.1097/AOG.0000000000002942
de Milliano I, Huirne JAF, Thurkow AL et al (2020) Ulipristal acetate vs gonadotropin-releasing hormone agonists prior to laparoscopic myomectomy (MYOMEX trial): short-term results of a double-blind randomized controlled trial. Acta Obstet Gynecol Scand 99(1):89–98. https://doi.org/10.1111/aogs.13713
Murji A, Wais M, Lee S, Pham A, Tai M, Liu G (2018) A multicenter study evaluating the effect of ulipristal acetate during myomectomy. J Minim Invasive Gynecol 25(3):514–521. https://doi.org/10.1016/j.jmig.2017.10.016
Chen BF, Powell MC, O’Beirne C (2017) An observation study of the clinical evaluation of symptom relief and side effects associated with taking ulipristal acetate (esmya) including its effect on pre-menstrual syndrome. J Obstet Gynaecol 37(5):645–648. https://doi.org/10.1080/01443615.2017.1287687
Luketic L, Shirreff L, Kives S et al (2017) Does ulipristal acetate affect surgical experience at laparoscopic myomectomy? J Minim Invasive Gynecol 24(5):797–802. https://doi.org/10.1016/j.jmig.2017.02.025
Verguts J, Godin PA, De Vree B et al (2019) Effect on surgical decisions: ulipristal acetate as key player in Belgian phase IV registration trial. Int J Gynaecol Obstet 147(3):339–343. https://doi.org/10.1002/ijgo.12968
Baggio S, Pomini P, Galeone F et al (2018) Influence of ulipristal acetate therapy on uterine fibroid-related symptoms and on uterine and fibroid volumes and vascularity indices assessed by ultrasound. J Ultrasound Med 37(9):2215–2223. https://doi.org/10.1002/jum.14573
Brun JL, Rajaonarison J, Froeliger A, Monseau-Thiburce AC, Randriamboavonjy R, Vogler A (2018) Outcome of patients with uterine fibroids after 3-month ulipristal acetate therapy. Eur J Obstet Gynecol Reprod Biol 222:13–18. https://doi.org/10.1016/j.ejogrb.2017.12.033
Yun BS, Seong SJ, Jung YW et al (2018) Predictive factor for volume reduction of uterine fibroids after short-term use of ulipristal acetate. Eur J Obstet Gynecol Reprod Biol 224:133–136. https://doi.org/10.1016/j.ejogrb.2018.03.026
Fernandez H, Schmidt T, Powell M, Costa AP, Arriagada P, Thaler C (2017) Real-world data of 1473 patients treated with ulipristal acetate for uterine fibroids: premya study results. Eur J Obstet Gynecol Reprod Biol 208:91–96. https://doi.org/10.1016/j.ejogrb.2016.11.003
Mallick R, Oxley S, Odejinmi F (2019) The use of ulipristal acetate (esmya) prior to laparoscopic myomectomy: help or hindrance? Gynecol Minim Invasive Ther 8(2):62–66. https://doi.org/10.4103/GMIT.GMIT_79_18
Ferrero S, Alessandri F, Vellone VG, Venturini PL, Leone Roberti Maggiore U (2016) Three-month treatment with ulipristal acetate prior to laparoscopic myomectomy of large uterine myomas: a retrospective study. Eur J Obstet Gynecol Reprod Biol 205:43–47. https://doi.org/10.1016/j.ejogrb.2016.08.021
Ferrero S, Racca A, Tafi E, Alessandri F, Venturini PL, Leone Roberti Maggiore U (2016) Ulipristal acetate before high complexity hysteroscopic myomectomy: a retrospective comparative study. J Minim Invasive Gynecol. 23(3):390–395. https://doi.org/10.1016/j.jmig.2015.12.002
Woodhead N, Pounds R, Irani S, Pradhan P (2018) Ulipristal acetate for uterine fibroids: 2 years of real-world experience in a UK hospital. J Obstet Gynaecol 38(6):813–817. https://doi.org/10.1080/01443615.2017.1405926
Lee MJ, Yun BS, Seong SJ et al (2017) Uterine fibroid shrinkage after short-term use of selective progesterone receptor modulator or gonadotropin-releasing hormone agonist. Obstet Gynecol Sci 60(1):69–73. https://doi.org/10.5468/ogs.2017.60.1.69
Donnez J, Hudecek R, Donnez O et al (2015) Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 103(2):519–27.e3. https://doi.org/10.1016/j.fertnstert.2014.10.038
Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review liver injury risk -European Medicines Agency (2020). Retrieved from https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk. Accessed 8 Apr 2020
Esmya (2018) Retrieved from https://www.ema.europa.eu/en/medicines/human/referrals/esmya#key-facts-section. Accessed 15 Nov 2020
Murji A, Whitaker L, Chow TL, Sobel ML (2017) Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010770.pub2 (Art. No.: CD010770)
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NS: protocol development, data collection, quality assessment, data analysis, manuscript writing. EE: protocol development, data collection, quality assessment, manuscript editing. MS: protocol development, data analysis, manuscript editing. FO: protocol development, manuscript editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, N., Egbase, E., Sideris, M. et al. What happens after randomised controlled trials? Uterine fibroids and ulipristal acetate: systematic review and meta-analysis of "real-world" data. Arch Gynecol Obstet 303, 1121–1130 (2021). https://doi.org/10.1007/s00404-020-05918-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05918-3