Abstract
Background
Amniotic fluid abnormalities may be associated with adverse perinatal outcomes, some of which are endocrine related.
Objective
To evaluate whether in utero exposure to amniotic fluid abnormalities is associated with long-term endocrine morbidity in the offspring.
Study design
In this cohort study, the incidence of long-term endocrine disorders was compared between singletons exposed and non-exposed to oligohydramnios or polyhydramnios.
Results
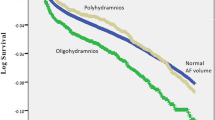
During the study period, 195 943 newborns were included in the study, of them 2.0% (n = 4072) and 2.9% (n = 5684) were exposed to oligohydramnios and polyhydramnios, respectively. Long-term endocrine morbidity was higher among children exposed to isolated amniotic fluid disorders, as was also noted in the Kaplan–Meier survival curve (log-rank test p < 0.001). Abnormal amniotic fluid volume was found to be independently associated with long-term endocrine morbidity of the offspring according to a Cox regression model controlled for clinically related confounders.
Conclusion
In utero exposure to isolated amniotic fluid abnormalities is independently associated with long-term endocrine morbidity in the offspring.

Similar content being viewed by others

References
Brace RA (1997) Physiology of amniotic fluid volume regulation. Clin Obstet Gynecol 40:280–289
Hill LM, Breckle R, Wolfgram KR, O’Brien PC (1983) Oligohydramnios; ultrasonically detected incidence and subsequent foetal outcome. Am J Obstet Gynecol 147:407–410
Shipp TD, Bromley B, Pauker S et al (1996) Outcome of singleton pregnancies with severe oligohydramnios in the second and third trimesters. Ultrasound Obstet Gynecol 7:108
Feldman I, Friger M, Wiznitzer A et al (2009) Is oligohydramnios more common during the summer season? Arch Gynecol Obstet 280:3
Zhu XQ, Jiang SS, Zhu XJ et al (2009) Expression of aquaporin 1 and aquaporin 3 in fetal membranes and placenta in human term pregnancies with oligohydramnios. Placenta 30:670
Touboul C, Picone O, Levaillant JM et al (2009) Clinical application of fetal urine production rate in unexplained polyhydramnios. Ultrasound Obstet Gynecol 34:521
Abele H, Starz S, Hoopmann M et al (2012) Idiopathic polyhydramnios and postnatal abnormalities. Fetal Diagn Ther 32:251
Dorleijn DM, Cohen-Overbeek TE, Groenendaal F et al (2009) Idiopathic polyhydramnios and postnatal findings. J Matern Fetal Neonatal Med 22:315
Laghmani K, Beck BB, Yang SS et al (2016) Polyhydramnios, transient antenatal bartter’s syndrome, and MAGED2 mutations. N Engl J Med 374:1853
Odibo IN, Newville TM, Ounpraseuth ST et al (2016) Idiopathic polyhydramnios: persistence across gestation and impact on pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol 199:175
Harlev A, Sheiner E, Friger M, Hershkovitz R (2014) Polyhydramnios and adverse perinatal outcome—what is the actual cutoff? J Matern Fetal Neonatal Med 27(12):1199–1203
Aviram A, Salzer L, Hiersch L et al (2015) Association of isolated polyhydramnios at or beyond 34 weeks of gestation and pregnancy outcome. Obstet Gynecol 125(4):825–832
Maymon E, Ghezzi F, Shoham-Vardi I et al (1998) Isolated hydramnios at term gestation and the occurrence of peripartum complications. Eur J Obstet Gynecol Reprod Biol 77(2):157–161
Klaassen I, Neuhaus TJ, Mueller-Wiefel DE, Kemper MJ (2007) Antenatal oligohydramnios of renal origin: long-term outcome. Nephrol Dial Transplant 22(2):432–439
Morris RK, Kilby MD (2011) Long-term renal and neurodevelopmental outcome in infants with LUTO, with and without fetal intervention. Early Hum Dev 87(9):607–610
Agarwal MM, Dhatt GS, Othman Y (2015) Gestational diabetes mellitus prevalence: effect of the laboratory analytical variation. Diabetes Res Clin Pract 109(3):493–499
Donovan L, Hartling L, Muise M, Guthrie A, Vandermeer B, Dryden DM (2013) Screening tests for gestational diabetes: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 159(2):115–122
Central Bureau of Statistics. Statistical abstract of Israel 2013, population by district, subdistrict and religion. https://www1.cbs.gov.il/reader/?MIval¼cw_usr_view_SHTML&ID¼807. Accessed Jan 2017
Strauss RS, Bradley LJ, Brolin RE (2001) Gastric bypass surgery in adolescents with morbid obesity. J Pediatr 138(4):499–504
Ogden CL, Carroll MD, Kit BK, Flegal KM (2014) Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311(8):806–814
Davis EF, Lazdam M, Lewandowski AJ et al (2012) Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 129(6):e1552–e1561
Lau EY, Liu J, Archer E, McDonald SM, Liu J (2014) Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J Obes 2014:524939
Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG et al (2011) Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev 12(7):525–542
Harper LM, Jauk VC, Owen J, Biggio JR (2014) The utility of ultrasound surveillance of fluid and growth in obese women. Am J Obstet Gynecol 211(5):524.e1–524.e8
Taveras EM, Rifas-Shiman SL, Sherry B et al (2011) Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med 165(11):993–998
Silverman BL, Metzger BE, Cho NH, Loeb CA (1995) Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care 18:611–617
Landon MB, Rice MM, Varner MW, Casey BM, Reddy UM, Wapner RJ et al (2015) Mild gestational diabetes mellitus and long-term child health. Diabetes Care 38(3):445–452
Zhang S, Regnault TR, Barker PL, Botting KJ, McMillen IC, McMillan CM et al (2015) Placental adaptations in growth restriction. Nutrients 2015(7):360–389
Stavrou S, Nicolaides NC, Critselis E, Darviri C, Charmandari E, Chrousos GP (2017) Pediatric stress: from neuroendocrinology to contemporary disorders. Eur J Clin Investig 47:262–269 [Epub ahead of print]
Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM (1993) Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36(1):62–67
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
PG: Study design, data interpretation. WA: Study design, data interpretation. WT: Data collection, data analysis, generation of figures. LD: Pediatric consultation. SR: Data collection, data analysis. SE: Study design, data interpretation. All authors were involved in writing the paper and had final approval of the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
Gali Pariente declares that she has no conflict of interests, Asnat Walfisch declares that she has no conflict of interests, Tamar Wainstock declares that she has no conflict of interests, Daniella Landau declares that she has no conflict of interests, Ruslan Sergienko declares that he has no conflict of interests, Eyal Sheiner declares that he has no conflict of interests.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was not needed to be obtained from individual participants included in the study as the individual identity was not known to the study researchers at any time.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pariente, G., Walfisch, A., Wainstock, T. et al. Prenatal exposure to isolated amniotic fluid disorders and the risk for long-term endocrine morbidity of the offspring. Arch Gynecol Obstet 302, 873–878 (2020). https://doi.org/10.1007/s00404-020-05674-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05674-4