Abstract
Background
Cytological analysis of ascitic fluid is an important tool for diagnosis, staging, and prognostic assessment in patients with cancer, but more detailed information is needed regarding malignancy rates and the time sequence in which ascites develops for different sites of cancer origin. Especially, an increased early tumor diagnosis may improve the acceptance for cytological examinations for the tumor patients in oncological practice.
Methods
Ascites specimens from patients who were treated at Bayreuth Hospital from 2006 to 2015 were reevaluated retrospectively and correlated with clinical reports.
Results
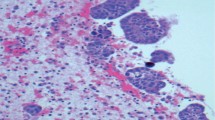
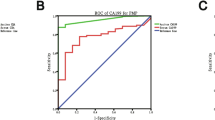
580 of all 941 ascitis specimens (61.6%) were from patients with malignancies with predominant appearance of gastrointestinal and gynecological tumors in 516/580 (89%) patients. Histologically, 549 (94.6%) were carcinomas, 23 (4%) hematological malignancies, 5 (0.9%) mesotheliomas and 3 (0.5%) were melanomas. Malignant ascitic fluid was noted in 298 of the 580 (51.4%) patients with cancer, thus the overall malignancy rate in the ascites specimens examined was 298/941 (31.7%). The most frequent malignancy rate for gynecological tumors we obtained in ovarian cancer with 85.7% and in the upper gastrointestinal tract with 77.8% for Barrett’s carcinoma and 61,4% for gastric carcinoma. Regarding time of detection, malignant ascitic fluid was noted as a separate finding, prior or simultaneous to the histological diagnosis of cancer in 225/298 patients (75.5%). An outstanding earliest occurrence was found in ovarian carcinoma in 94.9% and in the gastrointestinal tract in pancreatic carcinoma in 66.7%.
Conclusions
Tumor staging was the main important clinical question in our single center study of ascitic fluid, especially for patients with gastrointestinal and gynecological malignomas. The highest malignancy rate and earliest time of tumor detection caused the leading importance for ovarian tumors in malignant ascitic fluid. Moreover, the application of immunostains in our study allowed in 75.5% of all tumor patients a correct initial diagnosis, which is important for further clinical therapy.

















Similar content being viewed by others
Change history
30 May 2020
Unfortunately we did not explain the type of sample collection in ���materials and methods���.
References
Lengyel E (2010) Ovarian cancer development and metastasis. Am J Pathol 177(3):1053–1064. https://doi.org/10.2353/ajpath.2010.100105
Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL (2006) Metastatic patterns in adenocarcinoma. Cancer 106(7):1624–1633. https://doi.org/10.1002/cncr.21778
Robert Koch Institut. Zentrum für Krebsregisterdaten (2017) Eierstockkrebs (Ovarialkarzinom). https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Ovarialkrebs/ovarialkrebs.html. Accessed 16 Mar 2018
Quirk JT, Natarajan N (2005) Ovarian cancer incidence in the United States, 1992–1999. Gynecol Oncol 97(2):519–523. https://doi.org/10.1016/j.ygyno.2005.02.007
Salani R, Bristow RE (2012) Surgical management of epithelial ovarian cancer. Clin Obstet Gynecol 55(1):75–95. https://doi.org/10.1097/GRF.0b013e31824b4629
Wright AA, Bohlke K, Edelson MI (2016) Neoadjuvant chemotherapy for newly diagnosed advanced ovarian cancer: Society of Gynecologic Oncology and ASCO clinical practice guideline summary. J Oncol Pract 12(12):1254–1257. https://doi.org/10.1200/JOP.2016.016873
Runyon BA, Hoefs JC, Morgan TR (1988) Ascitic fluid analysis in malignancy-related ascites. Hepatology 8(5):1104–1109
Parsons SL, Watson SA, Steele RJ (1996) Malignant ascites. Br J Surg 83(1):6–14
Sangisetty SL, Miner TJ (2012) Malignant ascites: a review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg 4(4):87–95. https://doi.org/10.4240/wjgs.v4.i4.87
Runyon BA, Hoefs JC (1986) Peritoneal lymphomatosis with ascites. A characterization. Arch Intern Med 146(5):887–888
Krugmann J, Melcher B, Deuerling J, Mühldorfer S, Vieth M (2017) Klinisch-zytologische Korrelation der Aszitesdiagnostik bei gastrointestinalen Erkrankungen am Klinikum Bayreuth-eine retrospektive Untersuchung an 256 Patienten. Verdauungskrankheiten 35(3):129–139. https://doi.org/10.5414/VDX0950
Fiegl M, Massoner A, Haun M, Sturm W, Kaufmann H, Hack R, Krugmann J, Fritzer-Szekeres M, Grünewald K, Gastl G (2004) Sensitive detection of tumour cells in effusions by combining cytology and fluorescence in situ hybridisation (FISH). Br J Cancer 91(3):558–563. https://doi.org/10.1038/sj.bjc6601942
R Core Team 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ayantunde AA, Parsons SL (2007) Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol 18:945–949. https://doi.org/10.1093/annonc/mdl499
Garrison RN, Kaelin LD, Galloway RH, Heuser LS (1986) Malignant ascites. Clinical and experimental observations. Ann Surg 203(6):644–651
Halkia E, Chrelias G, Chrelias C, Esquivel J (2018) 2017 Update on ovarian cancer peritoneal carcinomatosis multimodal treatment considerations. Gastroenterol Res Pract. https://doi.org/10.1155/2018/5284814(eCollection 2018)
Sugarbaker PH (2018) Peritoneal metastasis. A frontier for progress. Surg Oncol Clin N Am 27(3):413–424. https://doi.org/10.1016/j.soc.2018.02.001
Arikan SK, Kasap B, Yetimalar H, Yildiz A, Sakarya DK, Tatar S (2014) Impact of prognostic factors on survival rates in patients with ovarian carcinoma. Asian Pac J Cancer Prev 15(15):6087–6094
Krugmann J, Lang-Schwarz C, Melcher B, Sterlacci W, Ozalinskaite A, Agaimy A, Vieth M (2018) Malignant ascites occurs most often in patients with high grade serous papillary ovarian cancer at initial diagnosis: a retrospective analysis of 191 women treated at Bayreuth hospital, 2006–2015. Arch Gynecol Obstet. https://doi.org/10.1007/s00404-18-4952-9
Prat J; FIGO Committee on Gynecologic Oncology (2015) FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol 26(2):87–89. https://doi.org/10.3802/jgo.2015.26.2.87
Shih IM, Kurman RJ (2004) Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164(5):1511–1518
Berek JS, Kehoe ST, Kumar L, Friedlander M (2018) Cancer of the ovary, fallopian tube, and peritonaeum. Int J Gynecol Obstet Suppl. https://doi.org/10.1002/ijgo.12614
Tuthill M, Pell R, Guillani R, Lim A, Gudi M, Contractor KB, Lewis JS, Coombes RC, Stebbing J (2009) Peritoneal disease in breast cancer: a specific entity with an extremely poor prognosis. Eur J Cancer 45(12):2146–2149. https://doi.org/10.1016/j.ejca2009.04.027
Robert Koch Institut. Zentrum für Krebsregisterdaten (2017) Gebärmutterhalskrebs. https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/gebärmutterhalskrebs/gebärmutterhalskrebs/html. Accessed 8 Nov 2018
Mulvani N (1996) Cytohistologic correlation in malignant peritoneal washings. Analysis of 75 malignant fluids. Acta Cytol 40(6):1231–1239. https://doi.org/10.1159/000333986
Hirasawa T, Yasuda M, Muramatsu T, Itoh H, Shinozuka T, Makino T, Tsutsumi Y, Osamura RY (1997) Cytologic study of ascites and the endometrium in ovarian carcinoma. Clinical significance. Acta Cytol 41(5):1451–1455. https://doi.org/10.1159/000332858
Kraemer O, Rapiti E, Huber D, Lopes-Raimundo E, Usel M, Bouchardy C, Petignat P (2015) Stage IVB endometrial cancer: clinical course and survival of patients with single and multiple metastasis. Eur J Gynecol Oncol 36(5):529–532
Kurita T, Matsuura Y, Koi C, Kagami S, Kawagoe T, Hachisuga T (2015) The relationship between positive peritoneal cytology and the prognosis of patients with uterine cervical cancer. Acta Cytol 59(2):201–206. https://doi.org/10.1159/000382068
Ditto A, Martinelli F, Carciangu M, Lorusso D, Raspagliesi F (2015) Peritoneal cytology as prognostic factor in cervical cancer. Diagn Cytopathol 43(9):705–709. https://doi.org/10.1002/dc.23276
Huang CC, Michael CW (2014) Cytomorphological features of metastatic squamous cell carcinoma in serous effusions. Cytopathology 25(2):112–119. https://doi.org/10.1111/cyt.12069
Le Phong C, Hubbard EW, Van Meter S, Nodit L (2017) Squamous cell carcinoma in serous effusions: avoiding pitfalls in this rare encounter. Diagn Cytopathol 45(12):1095–1099. https://doi.org/10.1002/dc.23827
Li D, Hu B, Zhou Y, Wan T, Si X (2018) Impact of tumor size on survival of patients with resected pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. BMC Cancer 18(1):985. https://doi.org/10.1186/s12885-018-4901-9
Hicks AM, Chou J, Capanu M, Lovery MA, You KH, O’Reilly EM (2016) Pancreas adenocarcinoma: ascites, clinical manifestations, and managements implications. Clin Colorectal Cancer 15(4):360–368. https://doi.org/10.1016/j.clcc2016.04.014
Enblad M, Graf W, Birgisson H (2018) Risk factors for appendiceal and colorectal peritoneal metastasis. Eur J Surg Oncol 44(7):997–1005. https://doi.org/10.1016/ejso2018.02.245
Huang CA, Attele A, Michael CW (2013) Cytomorphologic features of metastatic urothelial carcinoma in serous effusions. Diagn Cytopathol 41(7):569–574. https://doi.org/10.1002/dc.22896
Monappa V, Reddy SM, Kudva R (2018) Hematolymphoid neoplasms in effusion cytology. Cytojournal. https://doi.org/10.4103/cytojournal.cytojournal_48_17
Das DK (2006) Serous effusions in malignant lymphomas: a review. Diagn Cytopathol 34(5):335–347. https://doi.org/10.1002/dc.20432
El-Fattah MA (2017) Clinical characteristics and survival outcome of primary effusion lymphoma: a review of 105 patients. Hematol Oncol 35(4):878–883. https://doi.org/10.1002/hon.2372
Shimada K, Hayakawa F, Kiyoi H (2018) Biology and management of primary effusion lymphoma. Blood 132(18):1879–1888. https://doi.org/10.1182/blood-2018-03-791426
Amin W, Linkov F, Landsittel DP, Silverstein JC, Becich MJ (2018) Factors influencing malignant mesothelioma survival: a retrospective review of the national mesothelioma virtual bank cohort. F1000Res. https://doi.org/10.12688/f1000research.15512.1
Goldblum J, Hart WR (1995) Localized and diffuse mesotheliomas of the genital tract and peritoneum in women: a clinicopathologic study of nineteen true mesothelial neoplasms, other than adenomatoid tumors, multicystic mesotheliomas, and localized fibrous tumors. Am J Surg Pathol 19(10):1124–1137
Malpica A, Sant Ambrogio S, Deavers MT, Silva EG (2012) Well differentiated papillary mesothelioma of the female peritoneum. A clinicopathologic study of 26 cases. Am J Surg Pathol 36(1):117–127. https://doi.org/10.1097/PAS0b013e8132354a79
Onofre FB, Onofre AS, Pomjanski N, Buckstegge B, Grote HJ, Böckin A (2006) 9p21 deletion in the diagnosis of malignant mesothelioma in serous effusions additional immunocytochemistry, DNA-ICM, and AgNOR analysis. Cancer 114(3):204–215. https://doi.org/10.1002/cncr.23413
Hida T, Matsumoto S, Hamasaki M, Kawahara K, Tsujimura T, Hiroshima K, Kamei T, Taguchi K, Iwasaki A, Oda Y, Honda H, Nabeshima K (2015) Deletion status of p16 in effusion smear preparation correlates with that of underlying malignant pleural mesothelioma tissue. Cancer Sci 106(11):1635–1641. https://doi.org/10.1111/cas.12769
Acknowledgements
Sophia Rösch’s contribution to this study was made in partial fulfillment of the requirements for obtaining the degree of doctor of medicine. Parts of the study published here were used for her doctoral thesis at the Medical Faculty of Friedrich Alexander University of Erlangen–Nuremberg.
Author information
Authors and Affiliations
Contributions
JK: project development, data collection, data analysis, manuscript writing; CLS: data collection, data analysis; BM: data collection, manuscript writing; WS: data analysis, manuscript editing; SR: data collection; JL: project development, manuscript editing; MV: project development, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krugmann, J., Schwarz, C.L., Melcher, B. et al. Diagnostic impact of ascites cytology in 941 patients: malignancy rates and time of detection in ovarian cancer relative to other tumor types. Arch Gynecol Obstet 301, 1521–1532 (2020). https://doi.org/10.1007/s00404-020-05553-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05553-y