Abstract
Purpose
To investigate the role and underlying mechanism of H19 in regulating angiogenic capacity of extravillous trophoblasts.
Methods
Gain and loss of function experiments were performed using a human first-trimester extravillous trophoblast (EVT) cell line, HTR-8/SVneo cells. H19 was overexpressed or knocked down in HTR-8 cells by transfecting plasmid harboring whole-length H19 sequence (pH19) or siRNA specially targeting H19, respectively (siH19). Cell migration and tube-formation assay were assessed in the indicated groups. Gene expression was detected by RT-qPCR, Western blot, and ELISA assay.
Results
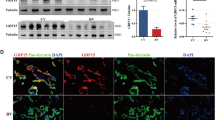
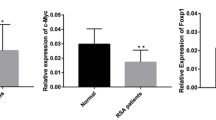
Overexpression of H19 in EVT cells increased cell migration and tube formation, while downregulation of H19 in EVT cells decreased cell migration and tube formation. Furthermore, we found that H19 played its role by VEGFA. In addition, we demonstrated the H19/miR-106a-5p/VEGFA regulatory axis in EVT. Experiments of the clinical specimen showed that H19 was very abundantly expressed in human first-trimester trophoblasts, and we found that the expression of H19 and VEGFA were significantly downregulated in the villous tissues from idiopathic recurrent miscarriage (RM) patients; moreover, the expression of H19 and VEGFA was positively correlated.
Conclusion
H19/miR-106a-5p/VEGFA axis plays a role in regulating the angiogenic capacity of EVT, which might contribute to idiopathic RM.





Similar content being viewed by others
References
He D, Zeng H, Chen J et al (2019) H19 regulates trophoblastic spheroid adhesion by competitively binding to let-7. Reprod 157(5):423–430
Zuckerwise L, Li J, Lu L et al (2016) H19 long noncoding RNA alters trophoblast cell migration and invasion by regulating TbetaR3 in placentae with fetal growth restriction. Oncotarget 7(25):38398–38407
Torry DS, Leavenworth J, Chang M et al (2007) Angiogenesis in implantation. J Assist Reprod Genet 24(7):303–315
Wulff C, Weigand M, Kreienberg R et al (2003) Angiogenesis during primate placentation in health and disease. Reproduction 126(5):569–577
Bischof P, Irminger-Finger I (2005) The human cytotrophoblastic cell, a mononuclear chameleon. Int J Biochem Cell Biol 37(1):1–16
Ishii T, Miyazawa M, Takanashi Y et al (2014) Genetically induced oxidative stress in mice causes thrombocytosis, splenomegaly and placental angiodysplasia that leads to recurrent abortion. Redox Biol 2:679–685
Banerjee P, Ghosh S, Dutta M et al (2013) Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. PLoS ONE 8(11):e80940
Zhu Y, Lu H, Huo Z et al (2016) MicroRNA-16 inhibits feto-maternal angiogenesis and causes recurrent spontaneous abortion by targeting vascular endothelial growth factor. Sci Rep 6:35536
Klauber N, Rohan RM, Flynn E et al (1997) Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nat Med 3(4):443–446
Meegdes BH, Ingenhoes R, Peeters LL et al (1988) Early pregnancy wastage: relationship between chorionic vascularization and embryonic development. Fertil Steril 49(2):216–220
Zeng H, Fan X, Liu N (2017) Expression of H19 imprinted gene in patients with repeated implantation failure during the window of implantation. Arch Gynecol Obstet 296(4):835–839
Poirier F, Chan CT, Timmons PM et al (1991) The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development 113(4):1105–1114
Xu J, Xia Y, Zhang H et al (2018) Overexpression of long non-coding RNA H19 promotes invasion and autophagy via the PI3K/AKT/mTOR pathways in trophoblast cells. Biomed Pharmacother 101:691–697
Jauniaux E, Poston L, Burton GJ (2006) Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update 12(6):747–755
Voellenkle C, Garcia-Manteiga JM, Pedrotti S et al (2016) Implication of long noncoding RNAs in the endothelial cell response to hypoxia revealed by RNA-sequencing. Sci Rep 6(1):24141
Hou J, Wang L, Wu Q et al (2018) Long noncoding RNA H19 upregulates vascular endothelial growth factor A to enhance mesenchymal stem cells survival and angiogenic capacity by inhibiting miR-199a-5p. Stem Cell Res Ther 9(1):109
Zhang C, Li Q, Ren N et al (2015) Placental miR-106a~363 cluster is dysregulated in preeclamptic placenta. Placenta 36(2):250–252
Premlata K, Yanmin L, Carmen T et al (2013) The c-Myc-regulated microRNA-17~92 (miR-17~92) and miR-106a~363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol 33(9):1782–1796
Fang Y, Yu S, Ma Y et al (2013) Association of Dll4/notch and HIF-1a-VEGF signaling in the angiogenesis of missed abortion. PLoS ONE 8(8):e70667
Meher A, Sundrani D, Joshi S (2015) Maternal nutrition influences angiogenesis in the placenta through peroxisome proliferator activated receptors: a novel hypothesis. Mol Reprod Dev 82(10):726–734
De Falco S (2012) The discovery of placenta growth factor and its biological activity. Exp Mol Med 44(1):1–9
Hiratsuka S, Kataoka Y, Nakao K et al (2005) Vascular endothelial growth factor A (VEGF-A) is involved in guidance of VEGF receptor-positive cells to the anterior portion of early embryos. Mol Cell Biol 25(1):355–363
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25(4):581–611
Amirchaghmaghi E, Rezaei A, Moini A et al (2015) Gene expression analysis of VEGF and its receptors and assessment of its serum level in unexplained recurrent spontaneous abortion. Cell J 16(4):538–545
Kutluer G, Cicek NM, Moraloglu O et al (2012) Low VEGF expression in conceptus material and maternal serum AFP and beta-hCG levels as indicators of defective angiogenesis in first-trimester miscarriages. J Turk Ger Gynecol Assoc 13(2):111–117
Bagheri A, Kumar P, Kamath A et al (2017) Association of angiogenic cytokines (VEGF-A and VEGF-C) and clinical characteristic in women with unexplained recurrent miscarriage. Bratisl Lek Listy 118(5):258–264
Plaisier M, Dennert I, Rost E et al (2009) Decidual vascularization and the expression of angiogenic growth factors and proteases in first trimester spontaneous abortions. Hum Reprod 24(1):185–197
Vuorela P, Carpen O, Tulppala M et al (2000) VEGF, its receptors and the tie receptors in recurrent miscarriage. Mol Hum Reprod 6(3):276–282
Almawi WY, Saldanha FL, Mahmood NA et al (2013) Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum Reprod 28(10):2628–2635
Acknowledgements
We thank Nongzhang Chen for her kindly help of collecting villous tissues.
Funding
This study was funded by the National Natural Science Foundation of China (https://www.nsfc.gov.cn/) (Grant number 81571441).
Author information
Authors and Affiliations
Contributions
NL was involved in the project designing, project development and manuscript revising. HZ and DH contributed to the project designing, project development, data collection, data analysis and manuscript writing. HX, YZ, JH, and BJ contributed to project development and data analysis.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The project was approved by the ethical committee of Xiangya Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, H., He, D., Xie, H. et al. H19 regulates angiogenic capacity of extravillous trophoblasts by H19/miR-106a-5p/VEGFA axis. Arch Gynecol Obstet 301, 671–679 (2020). https://doi.org/10.1007/s00404-020-05469-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05469-7