Abstract
Purpose
To characterize the immune cell infiltrate associated with maternal vascular remodeling in 12 cases of cesarean hysterectomy from women with placenta accreta spectrum (PAS) disorder.
Methods
Myometrial vessels were evaluated by hematoxylin and eosin and immunohistochemistry stains on tissue microarrays that included samples from the myometrium, deep to the implantation site. Vessels were quantified based on physiologic conversion and immune cell infiltrates. Placental bed biopsies from cases of repeat cesarean section, and decidual vessels from delivered non-PAS placentas were used as controls.
Results
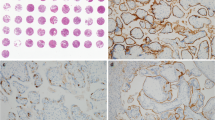
Immune cells, predominantly macrophages and T-cells, were present as a band along the placental-myometrial interface in PAS cases. However, within the myometrium, the infiltrate showed a perivascular accentuation. The infiltrates around and within vessel walls were composed of T-cells (CD3) and macrophages (CD68), with fewer NK (CD56), Treg (FoxP3) and rare B-cells (CD20). Plasma cells (CD138) were absent. The majority of vessels with immune cell infiltrates had undergone complete or partial physiologic conversion by trophoblast. However, a subset of unconverted vessels in the myometrium had a similar immune cell infiltration. Control blood vessels showed a similar pattern of leukocytes infiltration in the decidua and placental bed biopsies, but with a lower density.
Conclusions
Our findings suggest that myometrial vascular changes in PAS resemble the physiological changes of vessels noted in the implantation site of normal pregnancies. The presence of perivascular immune cell infiltrates in the absence of adjacent trophoblast suggests that the process may be initiated by paracrine effects rather than direct contact or endovascular growth of trophoblast.





Similar content being viewed by others
References
Tantbirojn P, Crum CP, Parast MM (2008) Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 29:639–645. https://doi.org/10.1016/j.placenta.2008.04.008
Jauniaux E, Burton GJ (2018) Pathophysiology of placenta accreta spectrum disorders: a review of current findings. Clin Obstet Gynecol 61:743–754. https://doi.org/10.1097/GRF.0000000000000392
Duzyj CM, Buhimschi IA, Motawea H, Laky CA, Cozzini G, Zhao G, Funai EF, Buhimschi CS (2015) The invasive phenotype of placenta accreta extravillous trophoblasts associates with loss of e-cadherin. Placenta 36:645–651. https://doi.org/10.1016/j.placenta.2015.04.001
Cramer SF, Heller D (2015) Placenta accreta and placenta increta—an approach to pathogenesis based on the trophoblastic differentiation pathway. Pediatr Dev Pathol. https://doi.org/10.2350/15-05-1641-OA.1
Suarez-Carmona M, Lesage J, Cataldo D, Gilles C (2017) Emt and inflammation: inseparable actors of cancer progression. Mol Oncol 11:805–823. https://doi.org/10.1002/1878-0261.12095
Vincent CT, Fuxe J (2017) Emt, inflammation and metastasis. Semin Cancer Biol 47:168–169. https://doi.org/10.1016/j.semcancer.2017.09.003
McMahon K, Karumanchi SA, Stillman IE, Cummings P, Patton D, Easterling T (2014) Does soluble fms-like tyrosine kinase-1 regulate placental invasion? Insight from the invasive placenta. Am J Obstet Gynecol 210(68):e61–64. https://doi.org/10.1016/j.ajog.2013.08.032
Khong TY, Robertson WB (1987) Placenta creta and placenta praevia creta. Placenta 8:399–409
Burton GJ, Woods AW, Jauniaux E, Kingdom JC (2009) Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30:473–482. https://doi.org/10.1016/j.placenta.2009.02.009
Harris LK, Benagiano M, D'Elios MM, Brosens I, Benagiano G (2019) Placental bed research: Ii. Functional and immunological investigations of the placental bed. Am J Obstet Gynecol 221:457–469. https://doi.org/10.1016/j.ajog.2019.07.010
Lee JY, Lee M, Lee SK (2011) Role of endometrial immune cells in implantation. Clin Exp Reprod Med 38:119–125. https://doi.org/10.5653/cerm.2011.38.3.119
Dannheim K, Shainker SA, Hecht JL (2016) Hysterectomy for placenta accreta; methods for gross and microscopic pathology examination. Arch Gynecol Obstet 293:951–958. https://doi.org/10.1007/s00404-015-4006-5
Richani K, Romero R, Kim YM, Cushenberry E, Soto E, Han YM, Espinoza J, Kim CJ (2006) Tissue microarray: an effective high-throughput method to study the placenta for clinical and research purposes. J Matern Fetal Neonatal Med 19:509–515. https://doi.org/10.1080/14767050600852718
Schneider CA, Rasband WS, Eliceiri KW (2012) Nih image to imagej: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Croy BA, Chen Z, Hofmann AP, Lord EM, Sedlacek AL, Gerber SA (2012) Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. Biol Reprod 87:125. https://doi.org/10.1095/biolreprod.112.102830
Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL (2009) Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 174:1959–1971. https://doi.org/10.2353/ajpath.2009.080995
DaSilva-Arnold SC, Zamudio S, Al-Khan A, Alvarez-Perez J, Mannion C, Koenig C, Luke D, Perez AM, Petroff M, Alvarez M, Illsley NP (2018) Human trophoblast epithelial-mesenchymal transition in abnormally invasive placenta. Biol Reprod 99:409–421. https://doi.org/10.1093/biolre/ioy042
Zhou Y, Genbacev O, Fisher SJ (2003) The human placenta remodels the uterus by using a combination of molecules that govern vasculogenesis or leukocyte extravasation. Ann NY Acad Sci 995:73–83
Tilburgs T, Crespo AC, van der Zwan A, Rybalov B, Raj T, Stranger B, Gardner L, Moffett A, Strominger JL (2015) Human hla-g+ extravillous trophoblasts: immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci USA 112:7219–7224. https://doi.org/10.1073/pnas.1507977112
Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL (2007) Tgfbeta promotes conversion of cd16+ peripheral blood nk cells into cd16− nk cells with similarities to decidual nk cells. Proc Natl Acad Sci U S A 104:3378–3383. https://doi.org/10.1073/pnas.0611098104
Khong TY, Bendon RW, Qureshi F, Redline RW, Gould S, Stallmach T, Lipsett J, Staples A (2000) Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol 31:292–295
Kim CJ, Romero R, Chaemsaithong P, Kim JS (2015) Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 213:S53–69. https://doi.org/10.1016/j.ajog.2015.08.041
Ernst LM, Linn RL, Minturn L, Miller ES (2017) Placental pathologic associations with morbidly adherent placenta: potential insights into pathogenesis. Pediatr Dev Pathol 20:387–393. https://doi.org/10.1177/1093526617698600
Schwede S, Alfer J, von Rango U (2014) Differences in regulatory T-cell and dendritic cell pattern in decidual tissue of placenta accreta/increta cases. Placenta 35:378–385. https://doi.org/10.1016/j.placenta.2014.03.004
Bailit JL, Grobman WA, Rice MM, Reddy UM, Wapner RJ, Varner MW, Leveno KJ, Iams JD, Tita AT, Saade G, Rouse DJ, Blackwell SC, Eunice Kennedy Shriver National Institute of Child H, Human Development Maternal-Fetal Medicine Units N (2015) Morbidly adherent placenta treatments and outcomes. Obstet Gynecol 125:683–689. https://doi.org/10.1097/AOG.0000000000000680
Balayla J, Bondarenko HD (2013) Placenta accreta and the risk of adverse maternal and neonatal outcomes. J Perinat Med 41:141–149. https://doi.org/10.1515/jpm-2012-0219
Acknowledgements
Immunohistochemistry was performed by the Specialized Histopathology Core at Dana-Farber Cancer Institute (Teri Bowman HT ASCP, Manager); and Beth Israel Deaconess Medical Center Pathology Department (Tonora Archibald, lead technologist). Specimen grossing was largely performed by lead technologist David Lamb (Beth Israel Deaconess Medical Center Pathology). We thank Saira Salahuddin for help with recruitment of subjects for this study.
Funding
This work was partially supported by the Charles Koch Foundation. The funder played no role in research design or implementation.
Author information
Authors and Affiliations
Contributions
JLH: data collection or management, data analysis, manuscript writing/editing. SAK: manuscript writing/editing. SAS: data collection or management, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from individual participants who were included as cases (those with disease) and placental bed biopsy controls in the study. Control samples of decidua were taken from delivered placentas collected during routine clinical practice, based on gestational age, after being de-identified for this study. These controls samples were obtained on a ‘discarded tissue’ protocol, with a waiver of informed consent from the institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hecht, J.L., Karumanchi, S.A. & Shainker, S.A. Immune cell infiltrate at the utero-placental interface is altered in placenta accreta spectrum disorders. Arch Gynecol Obstet 301, 499–507 (2020). https://doi.org/10.1007/s00404-020-05453-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05453-1