Abstract
Purpose
Gestational diabetes mellitus (GDM) and preeclampsia are leading causes of mortality and morbidity in mothers and children. High childhood body mass index (BMI) is among their myriad of negative outcomes. However, little is known about the trajectory of the child BMI exposed to GDM and co-occurring preeclampsia from early to mid-childhood. This study examined the independent and joint impact of GDM and preeclampsia on childhood BMI trajectory.
Methods
A population-based sample of 356 mothers were recruited from OB/GYN clinics in New York. Their children were then followed annually from 18 to 72 months. Maternal GDM and preeclampsia status were obtained from medical records. Child BMI was calculated based on their height and weight at annual visits.
Results
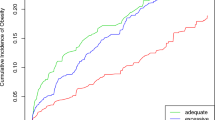
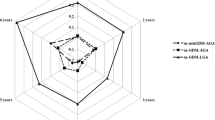
Hierarchical Linear Modeling was used to evaluate the trajectories of child BMI exposed to GDM and preeclampsia. BMI trajectory by GDM decreased (t ratio = − 2.24, \(\beta =\)0.45, 95% CI − 0.05–0.95, p = 0.07), but the trajectory by preeclampsia increased over time (t ratio = 3.153,\(\beta =\)0.65, 95% CI 0.11–1.18, p = 0.002). Moreover, there was a significant interaction between the two (t ratio = −2.24, \(\beta =\)− 1.244, 95% CI 0.15–2.33, p = 0.02), such that the BMI of children born to mothers with both GDM and preeclampsia showed consistent increases over time.
Conclusions
GDM and preeclampsia could be used as a marker for childhood obesity risk and the identification of a high-risk group, providing potential early intervention. These findings highlight the importance of managing obstetric complications, as an effective method of child obesity prevention.


Similar content being viewed by others
References
Hales CM, Carroll MD, Fryar CD et al (2017) Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 288:1–8
Reilly JJ, Armstrong J, Dorosty AR et al (2005) Early life risk factors for obesity in childhood: cohort study. BMJ 330(7504):1357
Schaefer-Graf UM, Pawliczak J, Passow D et al (2005) Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care 28(7):1745–1750
Zhou T, Sun D, Li et al (2018) Prevalence and trends in gestational diabetes mellitus among women in the United States, 2006–2016. Diabetes 67(Suppl 1)
ACOG Committee on Obstetric Practice (2018) ACOG practice bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol 131(2):e49–e64
DeSisto CL, Kim SY, Sharma AJ (2014) Prevalence estimates of gestational diabetes mellitus in the United States, pregnancy risk assessment monitoring system (prams). Prev Chronic Dis 11:E104
Dornhorst A, Paterson CM, Nicholls JSD et al (1992) High prevalence of gestational diabetes in women from ethnic minority groups. Diabet Med 9(9):820–825
ACOG Committee on Obstetric Practice (2019) ACOG Practice bulletin No. 202: diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol 133(1):e1–e25
Williams D (2011) Long-term complications of preeclampsia. Seminars in nephrology, vol 31. WB Saunders, Philadelphia, pp 111–122
Ornoy A (2011) Prenatal origin of obesity and their complications: gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 32(2):205–212
Mitanchez D (2010) Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab 36(6):617–662
Farahvar S, Walfisch A, Sheiner E (2019) Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expert Rev Endocrinol Metab 14(1):63–74
Nomura Y, Marks DJ, Grossman B et al (2012) Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention- deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med 166(4):337–343
Clausen TD, Mathiesen ER, Hansen T et al (2008) High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31(2):340–346
Abokaf H, Shoham-Vardi I, Sergienko R et al (2018) In utero exposure to gestational diabetes mellitus and long term endocrine morbidity of the offspring. Diabetes Res Clin Prac 144:231–235
Reece EA, Leguizamón G, Wiznitzer A (2009) Gestational diabetes: the need for a common ground. Lancet 373(9677):1789–1797
Cornier MA, Dabelea D, Hernandez TL et al (2008) The metabolic syndrome. Endocr Rev 29(7):777–822
Gillman MW, Rifas-Shiman S, Berkey CS et al (2003) Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 111(3):e221–e226
Hillier TA, Pedula KL, Schmidt MM et al (2007) Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 30(9):2287–2292
Kamana KC, Shakya S, Zhang H (2015) Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab 66(Suppl. 2):14–20
Ergaz Z, Avgil M, Ornoy A (2005) Intrauterine growth restriction—etiology and consequences: what do we know about the human situation and experimental animal models? Reprod Toxicol 20(3):301–322
Barker DJ, Hales CN, Fall CHD et al (1993) Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36(1):62–67
Hod M, Diamant YZ (1992) The offspring of a diabetic mother–short-and long-range implications. Isr J Med Sci 28(2):81–86
Ghulmiyyah L, Sibai B (2012) Maternal mortality from preeclampsia/eclampsia. Semin in Perinatol 36(1):56–59
Habli M, Levine RJ, Qian C et al (2007) Neonatal outcomes in pregnancies with preeclampsia or gestational hypertension and in normotensive pregnancies that delivered at 35, 36, or 37 weeks of gestation. AJOG 197(4):406.e1–406.e7
Masoura S, Kalogiannidis I, Margioula-Siarkou C et al (2012) Neonatal outcomes of late preterm deliveries with pre-eclampsia. Minerva Ginecol 64(2):109–115
Powe CE, Ecker J, Rana S et al (2011) Preeclampsia and the risk of large-for-gestational-age infants. AJOG 204(5):425.e1–425.e6
Bramham K, Briley AL, Seed P et al (2011) Adverse maternal and perinatal outcomes in women with previous preeclampsia: a prospective study. AJOG 204(6):512.e1–512.e9
Saadat M, Nejad SM, Habibi G et al (2007) Maternal and neonatal outcomes in women with preeclampsia. Taiwan J of Obstet & Gynecol 46(3):255–259
Forest JC, Girouard J, Massé J (2005) Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet & Gynecol 105(6):1373–1380
Palti H, Rothschild E (1989) Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum Dev 19(4):263–269
Seidman DS, Laor A, Gale R et al (1991) Pre-eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. BJOG 98(10):1009–1014
Tenhola S, Rahiala E, Martikainen A et al (2003) Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. The J of Clin Endocrinol & Metab 88(3):1217–1222
Davis EF, Lazdam M, Lewandowski AJ et al (2012) Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 129(6):e1552–e1561
Bos AF, Einspieler C, Prechtl HF (2001) Intrauterine growth retardation, general movements, and neurodevelopmental outcome: a review. Dev Med Child Neurol 43(1):61–68
Tolsa CB, Zimine S, Warfield SK et al (2004) Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res 56(1):132–138
Xiong X, Saunders LD, Wang FL et al (2001) Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J of Gynecol & Obstet 75(3):221–228
Li LJ, Aris IM, Su LL et al (2018) Effect of gestational diabetes and hypertensive disorders of pregnancy on postpartum cardiometabolic risk. Endocrin Connect 7(3):433–442
Kvehaugen AS, Andersen LF, Staff AC (2010) Anthropometry and cardiovascular risk factors in women and offspring after pregnancies complicated by preeclampsia or diabetes mellitus. Acta Obstet Gynecol Scand 89(11):1478–1485
Finik J, Nomura Y (2017) Cohort profile: stress in pregnancy (SIP) study. Int J of Epidemiol 46(5):1388
Maas CJ, Hox JJ (2004) The influence of violations of assumptions on multilevel parameter estimates and their standard errors. Comput Stat Data Anal 15;46(3):427–440
Rolland-Cachera MF, Deheeger M, Bellisle F et al (1984) Adiposity rebound in children: a simple indicator for predicting obesity. Am J of Clin Nutr 39:129–135
Langer O, Levy J, Brustman L et al (1989) Glycemic control in gestational diabetes mellitus-how tight is tight enough: small for gestational age versus large for gestational age? AJOG 161(3):646–653
Berkey CS, Rockett HR, Field AE et al (2000) Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics 105(4):e56
Gunderson EP, Greenspan LC, Faith MS et al (2018) SWIFT Offspring Study Investigators. Breastfeeding and growth during infancy among offspring of mothers with gestational diabetes mellitus: a prospective cohort study. Pediatr Obes 13(8):492–504
Miehle K, Stepan H, Fasshauer M (2012) Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol 76(1):2–11
Acknowledgements
The authors would like to thank the families for their participation, the Stress in Pregnancy laboratory staff at Queens College CUNY (especially Jackie Finik, Westar Zong, and Victoria Kuo), and the staff at the prenatal clinics and OB/GYN departments of Icahn School of Medicine at Mount Sinai and New York Presbyterian-Queens Hospital. This research work was supported by the National Institute of Mental Health under award number R01MH102729 and the Professional Staff Congress City University of New York grant to Y Nomura. The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This study was funded by the National Institute of Mental Health under award number R01MH102729 and the Professional Staff Congress City University of New York grant to Y Nomura.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. The first draft of the manuscript was written by 310 Yong Lin Huang and all authors commented on previous versions of the manuscript. All authors read and 311 approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research board committee of the City University of New York and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, Y., Zhang, W., Go, K. et al. Altered growth trajectory in children born to mothers with gestational diabetes mellitus and preeclampsia. Arch Gynecol Obstet 301, 151–159 (2020). https://doi.org/10.1007/s00404-020-05436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05436-2