Abstract
Background
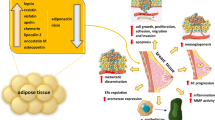
Type-II endometrial cancer is an estrogen independent and one of the most lethal types of cancer having poor prognosis. Adipokines play a crucial role in the triggering Type-II EMC. In addition, adipokines modulators, therefore, may have beneficial effects in the treatment of Type-II endometrial cancer, which was clinically evidenced.
Areas covered
This review presents the role of various adipokines involved and also the suitable modulators to treat Type-II endometrial cancer.
Conclusion
In the present review, we try to discuss the role of individual adipokines in the pathogenesis of Type-II endometrial cancer, and also the possible beneficial effects of adipokines modulator in the treatment of Type-II endometrial cancer.






Similar content being viewed by others
References
Reeves GK, Pirie K, Beral V et al (2007) Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 335:1134. https://doi.org/10.1136/bmj.39367.495995.AE
Schmandt RE, Iglesias DA, Co NN, Lu KH (2011) Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol 205:518–525. https://doi.org/10.1016/j.ajog.2011.05.042
Morice P, Leary A, Creutzberg C et al (2016) Endometrial cancer. Lancet 387:1094–1108. https://doi.org/10.1016/S0140-6736(15)00130-0
Saso S, Chatterjee J, Georgiou E et al (2011) Endometrial cancer. BMJ 343:d3954–d3954. https://doi.org/10.1136/bmj.d3954
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15:10–17
Key TJ, Appleby PN, Reeves GK et al (2003) Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95:1218–1226
Suh DH, Kim JW, Kang S et al (2014) Major clinical research advances in gynecologic cancer in 2013. J Gynecol Oncol 25:236–248. https://doi.org/10.3802/jgo.2014.25.3.236
Guan B, Mao TL, Panuganti PK et al (2011) Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol 35:625–632. https://doi.org/10.1097/PAS.0b013e318212782a
Prat J, Gallardo A, Cuatrecasas M, Catasús L (2007) Endometrial carcinoma: pathology and genetics. Pathology (Phila) 39:72–87. https://doi.org/10.1080/00313020601136153
Murali R, Soslow RA, Weigelt B (2014) Classification of endometrial carcinoma: more than two types. Lancet Oncol 15:e268–e278. https://doi.org/10.1016/S1470-2045(13)70591-6
Daley-Brown D, Oprea-Iles G, Vann KT et al (2017) Type II endometrial cancer overexpresses NILCO: a preliminary evaluation. Dis Markers 2017:1–14. https://doi.org/10.1155/2017/8248175
Beining RM, Dennis LK, Smith EM, Dokras A (2008) Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidemiol 18:492–499. https://doi.org/10.1016/j.annepidem.2007.11.011
Berstein LM, Santen RJ (2008) Innovative endocrinology of cancer. Springer, New York
Sakuragi N, Hirai A, Tada M et al (2001) Dominant-negative mutation of p53 tumor suppressor gene in endometrial carcinoma. Gynecol Oncol 83:485–490. https://doi.org/10.1006/gyno.2001.6429
Sakuragi N, Watari H, Ebina Y et al (2005) Functional analysis of p53 gene and the prognostic impact of dominant-negative p53 mutation in endometrial cancer. Int J Cancer 116:514–519. https://doi.org/10.1002/ijc.21097
Tashiro H, Isacson C, Levine R et al (1997) p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol 150:177–185
Lax SF, Kendall B, Tashiro H et al (2000) The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 88:814–824
Catasus L, D’Angelo E, Pons C et al (2010) Expression profiling of 22 genes involved in the PI3K-AKT pathway identifies two subgroups of high-grade endometrial carcinomas with different molecular alterations. Mod Pathol 23:694–702. https://doi.org/10.1038/modpathol.2010.44
Catasus L, Gallardo A, Cuatrecasas M, Prat J (2009) Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod Pathol 22:522–529. https://doi.org/10.1038/modpathol.2009.5
Shen F, Gao Y, Ding J, Chen Q (2017) Is the positivity of estrogen receptor or progesterone receptor different between type 1 and type 2 endometrial cancer? Oncotarget. https://doi.org/10.18632/oncotarget.13471
Oda K, Stokoe D, Taketani Y, McCormick F (2005) High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res 65:10669–10673. https://doi.org/10.1158/0008-5472.CAN-05-2620
Caduff RF, Johnston CM, Frank TS (1995) Mutations of the Ki-ras oncogene in carcinoma of the endometrium. Am J Pathol 146:182–188
Catasus L, Gallardo A, Cuatrecasas M, Prat J (2008) PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Mod Pathol 21:131–139. https://doi.org/10.1038/modpathol.3800992
Velasco A, Bussaglia E, Pallares J et al (2006) PIK3CA gene mutations in endometrial carcinoma: correlation with PTEN and K-RAS alterations. Hum Pathol 37:1465–1472. https://doi.org/10.1016/j.humpath.2006.05.007
Milam MR, Celestino J, Wu W et al (2007) Reduced progression of endometrial hyperplasia with oral mTOR inhibition in the Pten heterozygote murine model. Am J Obstet Gynecol 196:e1-247.e5. https://doi.org/10.1016/j.ajog.2006.10.872
Mutter GL, Lin MC, Fitzgerald JT et al (2000) Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 92:924–930
Djordjevic B, Hennessy BT, Li J et al (2012) Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod Pathol 25:699–708. https://doi.org/10.1038/modpathol.2011.208
Shen WH, Balajee AS, Wang J et al (2007) Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128:157–170. https://doi.org/10.1016/j.cell.2006.11.042
Fukuchi T, Sakamoto M, Tsuda H et al (1998) Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res 58:3526–3528
Palacios J, Catasús L, Moreno-Bueno G et al (2001) Beta- and gamma-catenin expression in endometrial carcinoma. Relationship with clinicopathological features and microsatellite instability. Virchows Arch Int J Pathol 438:464–469
Saegusa M, Hashimura M, Yoshida T, Okayasu I (2001) β-Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer 84:209–217. https://doi.org/10.1054/bjoc.2000.1581
Cheung LWT, Hennessy BT, Li J et al (2011) High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov 1:170–185. https://doi.org/10.1158/2159-8290.CD-11-0039
Wu H, Goel V, Haluska FG (2003) PTEN signaling pathways in melanoma. Oncogene 22:3113–3122. https://doi.org/10.1038/sj.onc.1206451
Rodriguez C, Calle EE, Fakhrabadi-Shokoohi D et al (2002) Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 11:822–828
Conroy MB, Sattelmair JR, Cook NR et al (2009) Physical activity, adiposity, and risk of endometrial cancer. Cancer Causes Control 20:1107–1115. https://doi.org/10.1007/s10552-009-9313-3
Schouten LJ, Goldbohm RA, van den Brandt PA (2004) Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst 96:1635–1638. https://doi.org/10.1093/jnci/djh291
Takeda K, Sagawa Y, Arakawa T (1970) Therapeutic effect of bleomycin for skin tumors. Gan 61:207–218
Conde J, Scotece M, Gómez R et al (2011) Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. BioFactors 37:413–420. https://doi.org/10.1002/biof.185
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625–1638. https://doi.org/10.1056/NEJMoa021423
Treas J, Tyagi T, Singh KP (2013) Chronic exposure to arsenic, estrogen, and their combination causes increased growth and transformation in human prostate epithelial cells potentially by hypermethylation-mediated silencing of MLH1: MLH1 Hypermethylation in Prostate Cancer. Prostate. https://doi.org/10.1002/pros.22701
Dossus L, Rinaldi S, Becker S et al (2010) Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer 17:1007–1019. https://doi.org/10.1677/ERC-10-0053
van Kruijsdijk RCM, van der Wall E, Visseren FLJ (2009) Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev 18:2569–2578. https://doi.org/10.1158/1055-9965.EPI-09-0372
Paz-Filho G, Lim EL, Wong ML, Licinio J (2011) Associations between adipokines and obesity-related cancer. Front Biosci Landmark Ed 16:1634–1650
Dedes KJ, Wetterskog D, Ashworth A et al (2011) Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol 8:261–271. https://doi.org/10.1038/nrclinonc.2010.216
Sengenès C, Miranville A, Lolmède K et al (2007) The role of endothelial cells in inflamed adipose tissue. J Intern Med 262:415–421. https://doi.org/10.1111/j.1365-2796.2007.01853.x
Dal Maso L, Augustin LSA, Karalis A et al (2004) Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab 89:1160–1163. https://doi.org/10.1210/jc.2003-031716
Siveen KS, Sikka S, Surana R et al (2014) Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta BBA Rev Cancer 1845:136–154. https://doi.org/10.1016/j.bbcan.2013.12.005
Wu Y, Zhou BP (2010) TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. Br J Cancer 102:639–644. https://doi.org/10.1038/sj.bjc.6605530
Völkl A, Ule G (1972) Trace elements in human brain. Iron concentration of 13 brain areas as a function of age. Z Neurol 202:331–338
Fujimoto H, Sangai T, Ishii G et al (2009) Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer 125:1276–1284. https://doi.org/10.1002/ijc.24378
He Z, Ong CHP, Halper J, Bateman A (2003) Progranulin is a mediator of the wound response. Nat Med 9:225–229. https://doi.org/10.1038/nm816
Tan BK, Adya R, Farhatullah S et al (2010) Metformin treatment may increase Omentin-1 levels in women with polycystic ovary syndrome. Diabetes 59:3023–3031. https://doi.org/10.2337/db10-0124
Qi D, Tang X, He J et al (2016) Omentin protects against LPS-induced ARDS through suppressing pulmonary inflammation and promoting endothelial barrier via an Akt/eNOS-dependent mechanism. Cell Death Dis 7:e2360–e2360. https://doi.org/10.1038/cddis.2016.265
Aleksandrova K, di Giuseppe R, Isermann B et al (2016) Circulating omentin as a novel biomarker for colorectal cancer risk: data from the EPIC–Potsdam cohort study. Cancer Res 76:3862–3871. https://doi.org/10.1158/0008-5472.CAN-15-3464
Blüher M (2012) Vaspin in obesity and diabetes: pathophysiological and clinical significance. Endocrine 41:176–182. https://doi.org/10.1007/s12020-011-9572-0
Friedman J (2014) 20 years of leptin: leptin at 20: an overview. J Endocrinol 223:T1-8. https://doi.org/10.1530/JOE-14-0405
Garofalo C, Surmacz E (2006) Leptin and cancer. J Cell Physiol 207:12–22. https://doi.org/10.1002/jcp.20472
Baratta M (2002) Leptin—from a signal of adiposity to a hormonal mediator in peripheral tissues. Med Sci Monit Int Med J Exp Clin Res 8:RA282–RA292
Bado A, Levasseur S, Attoub S et al (1998) The stomach is a source of leptin. Nature 394:790–793. https://doi.org/10.1038/29547
Frühbeck G (2006) Intracellular signalling pathways activated by leptin. Biochem J 393:7–20. https://doi.org/10.1042/BJ20051578
Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24:7455–7464. https://doi.org/10.1038/sj.onc.1209085
Huang W-S, Chen CN, Sze CI, Teng CC (2013) Visfatin induces stromal cell-derived factor-1 expression by β1 integrin signaling in colorectal cancer cells. J Cell Physiol 228:1017–1024. https://doi.org/10.1002/jcp.24248
Wang PP, He XY, Wang R et al (2014) High leptin level is an independent risk factor of endometrial cancer: a meta-analysis. Cell Physiol Biochem 34:1477–1484. https://doi.org/10.1159/000366352
Frankenberry KA, Skinner H, Somasundar P et al (2006) Leptin receptor expression and cell signaling in breast cancer. Int J Oncol 28:985–993
Soeda J, Cordero P, Li J et al (2017) Hepatic rhythmicity of endoplasmic reticulum stress is disrupted in perinatal and adult mice models of high-fat diet-induced obesity. Int J Food Sci Nutr 68:455–466. https://doi.org/10.1080/09637486.2016.1261086
T Jardé S Perrier MP Vasson F Caldefie-Chézet 2011 Molecular mechanisms of leptin and adiponectin in breast cancer Eur J Cancer Oxf Engl 47 33 43 10.1016/j.ejca.2010.09.005
Steppan CM, Bailey ST, Bhat S et al (2001) The hormone resistin links obesity to diabetes. Nature 409:307–312. https://doi.org/10.1038/35053000
Silswal N, Singh AK, Aruna B et al (2005) Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun 334:1092–1101. https://doi.org/10.1016/j.bbrc.2005.06.202
Kaser S, Kaser A, Sandhofer A et al (2003) Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun 309:286–290
Yadav A, Kumar B, Datta J et al (2011) IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res 9:1658–1667. https://doi.org/10.1158/1541-7786.MCR-11-0271
KF Yoong G McNab SG Hübscher DH Adams 1998 Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma J Immunol Baltim Md 160 3978 3988
Wai Wong C, Dye DE, Coombe DR (2012) The role of Immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J Cell Biol 2012:1–9. https://doi.org/10.1155/2012/340296
Johnson JP (1999) Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev 18:345–357
Hsieh YY, Shen CH, Huang WS et al (2014) Resistin-induced stromal cell-derived factor-1 expression through Toll-like receptor 4 and activation of p38 MAPK/NFκB signaling pathway in gastric cancer cells. J Biomed Sci 21:59. https://doi.org/10.1186/1423-0127-21-59
Hlavna M, Kohut L, Lipkova J et al (2011) Relationship of resistin levels with endometrial cancer risk. Neoplasma 58:124–128. https://doi.org/10.4149/neo_2011_02_124
Samal B, Sun Y, Stearns G et al (1994) Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol 14:1431–1437
Preiss J, Handler P (1957) Enzymatic synthesis of nicotinamide mononucleotide. J Biol Chem 225:759–770
Rongvaux A, Shea RJ, Mulks MH et al (2002) Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol 32:3225–3234. https://doi.org/10.1002/1521-4141(200211)32:11%3c3225:AID-IMMU3225%3e3.0.CO;2-L
Tian W, Zhu Y, Wang Y et al (2013) Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol Oncol 129:505–512. https://doi.org/10.1016/j.ygyno.2013.02.022
Jacques C, Holzenberger M, Mladenovic Z et al (2012) Proinflammatory actions of Visfatin/Nicotinamide phosphoribosyltransferase (Nampt) involve regulation of insulin signaling pathway and nampt enzymatic activity. J Biol Chem 287:15100–15108. https://doi.org/10.1074/jbc.M112.350215
Revollo JR, Grimm AA, Imai S (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279:50754–50763. https://doi.org/10.1074/jbc.M408388200
Tokmak A, Kokanali MK, Guzel AI et al (2014) Polycystic ovary syndrome and risk of endometrial cancer: a mini-review. Asian Pac J Cancer Prev APJCP 15:7011–7014
Jongwutiwes T, Lertvikool S, Leelaphiwat S et al (2009) Serum visfatin in Asian women with polycystic ovary syndrome. Gynecol Endocrinol 25:536–542. https://doi.org/10.1080/09513590903015478
Shackelford RE, Mayhall K, Maxwell NM et al (2013) Nicotinamide phosphoribosyltransferase in malignancy: a review. Genes Cancer 4:447–456. https://doi.org/10.1177/1947601913507576
Nowell MA, Richards PJ, Fielding CA et al (2006) Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum 54:2084–2095. https://doi.org/10.1002/art.21942
Buldak RJ, Gowarzewski M, Buldak L et al (2015) Viability and oxidative response of human colorectal HCT-116 cancer cells treated with visfatin/eNampt in vitro. J Physiol Pharmacol Off J Pol Physiol Soc 66:557–566
Cymbaluk-Płoska A, Chudecka-Głaz A, Pius-Sadowska E et al (2018) Circulating serum level of visfatin in patients with endometrial cancer. BioMed Res Int. https://doi.org/10.1155/2018/8576179
Nergiz Avcioglu S, Altinkaya SO, Küçük M et al (2015) Visfatin concentrations in patients with endometrial cancer. Gynecol Endocrinol 31:202–207. https://doi.org/10.3109/09513590.2014.975687
Hotta K, Funahashi T, Bodkin NL et al (2001) Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50:1126–1133
Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T (2014) Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab 28:15–23. https://doi.org/10.1016/j.beem.2013.09.003
Yamauchi T, Kamon J, Waki H et al (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946. https://doi.org/10.1038/90984
Yamauchi T, Kamon J, Ito Y et al (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769. https://doi.org/10.1038/nature01705
Holland WL, Miller RA, Wang ZV et al (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17:55–63. https://doi.org/10.1038/nm.2277
Petridou E, Mantzoros C, Dessypris N et al (2003) Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab 88:993–997. https://doi.org/10.1210/jc.2002-021209
Moon H-S, Chamberland JP, Aronis K et al (2011) Direct role of adiponectin and adiponectin receptors in endometrial cancer: in vitro and ex vivo studies in humans. Mol Cancer Ther 10:2234–2243. https://doi.org/10.1158/1535-7163.MCT-11-0545
Zeng F, Shi J, Long Y et al (2015) Adiponectin and endometrial cancer: a systematic review and meta-analysis. Cell Physiol Biochem 36:1670–1678. https://doi.org/10.1159/000430327
Hammarstedt A, Andersson CX, Rotter Sopasakis V, Smith U (2005) The effect of PPARγ ligands on the adipose tissue in insulin resistance. Prostaglandins Leukot Essent Fatty Acids 73:65–75. https://doi.org/10.1016/j.plefa.2005.04.008
Hammarstedt A, Pihlajamäki J, Rotter Sopasakis V et al (2006) Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J Clin Endocrinol Metab 91:1181–1184. https://doi.org/10.1210/jc.2005-1395
Törüner F, Akbay E, Çakır N, Sancak B, Elbeg Ş, Taneri F, Aktürk M, Karakoc A, Ayvaz G, Arslan M (2004) Effects of PPARγ and PPARα agonists on serum leptin levels in diet-induced obese rats. Horm Metab Res 36:226–230. https://doi.org/10.1055/s-2004-814452
Plaisance EP, Lukasova M, Offermanns S et al (2009) Niacin stimulates adiponectin secretion through the GPR109A receptor. Am J Physiol-Endocrinol Metab 296:E549–E558. https://doi.org/10.1152/ajpendo.91004.2008
Ando H, Sugimoto K, Yanagihara H et al (2008) Effects of atorvastatin and pravastatin on glucose tolerance, adipokine levels and inflammatory markers in hypercholesterolaemic patients. Clin Exp Pharmacol Physiol 35:1012–1017. https://doi.org/10.1111/j.1440-1681.2008.04945.x
Rossi AS, Lombardo YB, Lacorte JM et al (2005) Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am J Physiol-Regul Integr Comp Physiol 289:R486–R494. https://doi.org/10.1152/ajpregu.00846.2004
Funding
No funding is required for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The first author Garikapati Kusuma Kumari declares that she has no conflict of interest. The second author A. V. V. V. Ravi Kiran declares that he has no conflict of interest. The third author and corresponding author Dr. Praveen T. K declares that he has no conflict of interest. The fourth author Pavan Kumar Chintamaneni declares that he has no conflict of interest. The fifth author Sai Kiran S. S. Pindiprolu declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garikapati, K.K., Ammu, V.V.V.R.K., Krishnamurthy, P.T. et al. Type-II endometrial cancer: role of adipokines. Arch Gynecol Obstet 300, 239–249 (2019). https://doi.org/10.1007/s00404-019-05181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05181-1