Abstract
Objective
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women, involving hyperandrogenaemia and insulin resistance. Treatment options include dexamethasone, as well as the off-label use of metformin. To evaluate the impact of those drugs on cyclic changes in endometrial development, we tested possible effects of metformin and dexamethasone on endometrial stromal cells decidualisation, proliferation, and gene regulation in a hyperandrogenaemic microenvironment in vitro.
Methods/design
Ten endometrial biopsies (of which five were decidualized in vitro) were used from regularly cycling women. Cells were treated with testosterone, dexamethasone, and metformin in different concentrations. Thereafter, cells were assessed for proliferation and decidualization capacity, as well as mTor and MMP-2 gene regulation.
Results
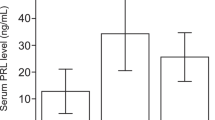
Metformin showed a dose-dependent negative effect on prolactin secretion, a known decidualization marker. This effect was stronger in a hyperandrogenaemic condition and could not be compensated by dexamethasone. Testosterone had a dose dependent negative effect on proliferation in decidualized endometrial stromal cells. Dexamethasone slightly compensated the negative proliferative effect only in low-dose testosterone. High-dose metformin also showed a dose-dependent reduction in endometrial stromal cell proliferation without a major impact by testosterone or dexamethasone in decidualized and non-decidualized cells. High-dose metformin significantly reduced the expression of matrix metalloproteinase-2 (MMP-2) and mechanistic Target of Rapamycin (mTor), regardless of the concentration of dexamethasone and testosterone. The strongest effect could be observed for the combination with high-dose dexamethasone.
Conclusion
When therapies, such as metformin and dexamethasone, are used to normalize peripheral androgen levels in patients with PCOS, their effect on the endometrial microenvironment should be taken into consideration as well, especially metformin has to be used with caution because of its dose dependent, possibly inhibiting effect at the endometrial proliferation.



Similar content being viewed by others
References
Hackbart KS, Cunha PM, Meyer KM, Wiltbank MC (2013) Effect of Glucocorticoid-Induced Insulin Resistance on Follicle Development and Ovulation. Biol Reprod 88(6):153 (1–12)
Huang Y, Li W, Wang CC, Wu X, Zheng J (2014) Cryptotanshinone reverses ovarian insulin resistance in mice through activation of insulin signaling and the regulation of glucose transporters and hormone synthesizing enzymes. Fertil Steril 102(2):589–596.e4
Tock L, Carneiro G, Pereira AZ, Tufik S, Zanella MT (2014) Adrenocortical production is associated with higher levels of luteinizing hormone in nonobese women with polycystic ovary syndrom. Int J Endocrinol 2014:620605. doi:10.1155/2014/620605
Tso LO, Costello MF, Albuquerque LET, Andriolo RB, Macedo CR (2014) Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. doi:10.1002/14651858.CD006105.pub3
Esmaeilzadeh S, Golsorkhtabar A, Basirat Z, Shirazi M (2011) Does Adding Dexamethasone to Clomiphene Citrate Improve Ovulation in PCOS Patients? A Triple—Blind Randomized Clinical Trial Study. Int J Fertil Steril 5:9–12
Heng S, Dynon K, Li Y, Edgell T, Rombauts LJ, Vollenhoven B and Nie G (2014) Development of a high throughput assay for human proprotein convertase 5/6 for detecting uterine receptivity. Anal Biochem. doi:10.1016/j.ab.2014.12.012
Timeva T, Shterev A, Kyurkchiev S (2014) Recurrent implantation failure: the role of the endometrium. J Reprod Infertil 15(4):173–183
Cha J, Sun X, Dey SK (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18(12):1754–1767. doi:10.1038/nm.3012
Schulte MM, Tsai J, Moley KH (2015) Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod Sci 22(1):6–14
Kim JY, Song H, Kim H, Kang HJ, Jun JH, Hong SR, Koong MK, Kim IS (2009) Transcriptional profiling with a pathway-oriented analysis identifies dysregulated molecular phenotypes in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab 94(4):1416–1426
Maliqueo MA, Quezada S, Clementi M, Bacallao K, Anido M, Johnson C, Vega M (2004) Potential action of androstenedione on the proliferation and apoptosis of stromal endometrial cells. Reprod Biol Endocrinol 10(2):81
Tuckerman EM, Okon MA, Li T, Laird SM (2000) Do androgens have a direct effect on endometrial function? An in vitro study. Fertil Steril 74(4):771–779
Okon MA, Laird SM, Tuckerman EM, Li TC (1998) Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril 69(4):682–690
Jalali Tehrani H, Parivar K, Ai J, Kajbafzadeh A, Rahbarghazi R, Hashemi M and Sadeghizadeh M (2014) Effect of dexamethasone, insulin and EGF on the myogenic potential on human endometrial stem cell. Iran J Pharm Res 13(2):659–664
Elnashar A, Abdelmageed E, Fayed M, Sharaf M (2006) Clomiphene citrate and dexamethazone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study. Hum Reprod 21(7):1805–1808
Davies S, Dai D, Pickett G, Leslie KK (2006) Gene regulation profiles by progesterone and dexamethasone in human endometrial cancer Ishikawa H cells. Gynecol Oncol 101(1):62–70
Makrigiannakis A, Margioris AN, Chatzaki E, Zoumakis E, Chrousos GP, Gravanis A (1999) The decidualizing effect of progesterone may involve direct transcriptional activation of corticotrophin-releasing hormone from human endometrial stromal cells. Mol Hum Reprod 5(9):789–96
Ibáñez L, Potau N, Marcos MV, de Zegher F (1999) Corticotropin-releasing hormone: a potent androgen secretagogue in girls with hyperandrogenism after precocious pubarche. J Clin Endocrinol Metab 84(12):4602–4606
Maia-Filho VO, Rocha AM, Ferreira FP, Bonetti TC, Serafini P, Motta EL (2014) Matrix metalloproteinases 2 and 9 and e-cadherin expression in the endometrium during the implantation window of infertile women before in vitro fertilization treatment. Reprod Sci 22(4):416–422
Van der Stehen PE, Dubois B, Inge Nelissen, Rudd PM, DWEK AR, Obdenakker G (2002) Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 37(6):375–536. doi:10.1080/10409230290771546
Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J (2001) Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol 69(6):851–859
Wang Y, Zhu L, Kuokkanen S, Pollard JW (2015) Activation of protein synthesis in mouse uterine epithelial cells by estradiol-17β is mediated by a PKC-ERK1/2-mTOR signaling pathway. Proc Natl Acad Sci USA 112(11):E1382–91
Yue X, Wu L, Hu W.(2015) The regulation of leukemia inhibitory factor. Cancer Cell Microenviron. doi:10.14800/ccm.877
Margioula-Siarkou C, Prapas Y, Petousis S, Milias S, Ravanos K, Kalogiannidis I, Mavromatidis G, Haitoglou C, Prapas N, Rousso D (2016) LIF and LIF-R expression in the endometrium of fertile and infertile women: a prospective observational case-control study. Mol Med Rep 13(6):4721–4728
Li X, Feng Y, Lin JF, Billig H, Shao R (2014) Endometrial progesterone resistance and PCOS. J Biomed Sci 21:2
Savaris RF, Groll JM, Young SL, DeMayo FJ, Jeong JW, Hamilton AE, Giudice LC, Lessey BA (2011) Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab 96(6):1737–1746
Fox C, Morin S, Jeong JW, Scott RT Jr, Lessey BA (2016) Local and systemic factors and implantation: what is the evidence? Fertil Steril 105(4):873–884
Vanky E, Salvesen KA, Carlsen SM (2004) Six-month treatment with low-dose dexamethasone further reduces androgen levels in PCOS women treated with diet and lifestyle advice, and metformin. Hum Reprod 3(3):529–533
Jung ML Renke T, Nowak O, Jauckus J, Zorn M, Capp E, Strowitzki T, Germeyer A (2015): Modulation of the IGF system and proliferation in human endometrial stromal cells by metformin: a dose-dependent effect. Arch Gynecol Obstet 292(2):465–472
Obara A, Fujita Y, Abudukadier A, Fukushima T, Oguri Y, Ogura M, Harashima S, Hosokawa M, Inagaki N (2015): DEPTOR-related mTOR suppression is involved in metformin’s anti-cancer action in human liver cancer cells. Biochem Biophys Res Commun 460(4):1047–1052
Shen C, Kim MR, Noh JM, Kim SJ, Ka SO, Kim JH, Park BH, Park JH (2016) Glucocorticoid Suppresses Connexin 43 Expression by Inhibiting the Akt/mTOR Signaling Pathway in Osteoblasts. Calcif Tissue Int 99:88–97
Legro RS (2016) Ovulation induction in polycystic ovary syndrome: current options. Best Pract Res Clin Obstet Gynaecol 37:152–159
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Merck Serono.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Freis, A., Renke, T., Kämmerer, U. et al. Effects of a hyperandrogenaemic state on the proliferation and decidualization potential in human endometrial stromal cells. Arch Gynecol Obstet 295, 1005–1013 (2017). https://doi.org/10.1007/s00404-017-4295-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4295-y