Abstract
Purpose
To determine if endometrial gene expression is different in women with endometriosis-related infertility and fertile women.
Methods
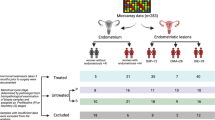
Prospective study of mid-follicular phase endometrium in 47 subjects in two phases: microarray study of 10 infertile women with endometriosis and five fertile controls, and a quantitative real-time PCR (qRT-PCR) study of 27 infertile women with endometriosis and 15 fertile controls. Gene expression was determined by DNA microarray, and qRT-PCR used for 12 “promising” genes based on the microarray analysis.
Results
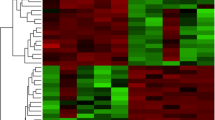
Compared to fertile controls, women with stage I–II endometriosis had 23, and women with stage III–IV had 35 genes that were significantly up- or down-regulated by microarray. However, using qRT-PCR, only chemokine ligand (CXCL) 13 was significantly down-regulated and somatostatin was significantly up-regulated with early endometriosis, and only CXCL 14 was significantly down-regulated with advanced endometriosis compared to fertile controls.
Conclusions
Our findings indicate that the pattern of gene expression in proliferative-phase endometrium is different when comparing tissue from patients with endometriosis versus fertile controls. Recognition of these endometrial alterations could be helpful to diagnose and stage endometriosis, and may provide insight to explain why conception rates are low in women with endometriosis.
Similar content being viewed by others
References
Mahmood TA, Templeton A (1991) Prevalence and genesis of endometriosis. Hum Reprod 6:544–549
Ishimaru T, Masuzaki H (1991) Peritoneal endometriosis: endometrial tissue implantation as its primary etiologic mechanism. Am J Obstet Gynecol 165:210–214
Oral E, Arici A (1997) Pathogenesis of endometriosis. Obstet Gynecol Clin North Am 24:219–233
Annunziata M, Luque RM, Duran-Prado M, Baragli A, Grande C, Volante M, Gahete MD, Deltetto F, Camanni M, Ghigo E, Castano JP, Granata R (2012) Somatostatin and somatostatin analogues reduce PDGF-induced endometrial cell proliferation and motility. Hum Reprod 27:2117–2129
Sharpe-Timms KL, Nabli H, Zimmer RL, Birt JA, Davis JW (2012) Inflammatory cytokines differentially up-regulate human endometrial haptoglobin production in women with endometriosis. Hum Reprod 25:1241–1250
Montagna P, Capellino S, Villaggio B, Remorgida V, Ragni N, Cutolo M, Ferrero S (2008) Peritoneal fluid macrophages in endometriosis: correlation between the expression of estrogen receptors and inflammation. Fertil Steril 90:156–164
Gazvani R, Templeton A (2002) Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 123:217–226
Bulun SE (2009) Endometriosis. N Engl J Med 360:268–279
Barnhart K, Dunsmoor-Su R, Coutifaris C (2002) Effect of endometriosis on in vitro fertilization. Fertil Steril 77:1148–1155
Pate JL, Toyokawa K, Walusimbi S, Brzezicka E (2010) The interface of the immune and reproductive systems in the ovary: lessons learned from the corpus luteum of domestic animal models. Am J Reprod Immunol 64:275–286
Hou Z, Sun L, Gao L, Liao L, Mao Y, Liu J (2009) Cytokine array analysis of peritoneal fluid between women with endometriosis of different stages and those without endometriosis. Biomarkers 14:604–618
Nishida M, Nasu K, Junichiro F, Yasushi K, Narahara H, Miyakawa I (2004) Down-regulation of interleukin-1 receptor type 1 expression causes the dysregulated expression of CXC chemokines in endometriotic stromal cells: a possible mechanism for the altered immunological functions in endometriosis. JCEM 89:5094–5100
Furuya M, Suyama T, Usui H, Kasuya Y, Mishiyama M, Tanaka N, Ishiwata I, Nagai Y, Shozu M, Kimura S (2007) Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol 38:1676–1687
Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT (1998) A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature 391:799–803
Starnes T, Rasila KK, Robertson MJ, Brahmi Z, Dahl R, Christopherson K, Hromas R (2006) The chemokine CXCL14 (BRAK) stimulates activated NK cell migration: implications for the downregulation of CXCL14 in malignancy. Exp Hematol 34:1101–1105
Kurth I, Willimann K, Schaerli P, Hunziker T, Clark-Lewis I, Moser B (2001) Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J Exp Med 194:855–861
Shurin GV, Ferris R, Tourkova IL, Perez L, Lokshin A, Balkir L, Collins B, Chatta GS, Shurin MR (2005) Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo. J Immun 174:5490–5498
Shellenberger TD, Wang M, Gujrati M, Jayakumar A, Strieter RM, Burdick MD, Ioannides CG, Efferson CL, El-Naggar AK, Roberts D, Clayman GL, Frederick MJ (2004) BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res 64:8262–8270
Acknowledgments
Funding was provided by a Carolinas Laparoscopic Advanced Surgical Program grant, Carolinas Medical Center, Charlotte, North Carolina; Research support was provided by the Kristen Bennett and other research staff at the Cannon Research Center, including sample preparation, data analysis, and qPCR.
Conflict of interest
The authors report no declarations of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hurst, B.S., Shimp, K.E., Elliot, M. et al. Molecular evaluation of proliferative-phase endometrium may provide insight about the underlying causes of infertility in women with endometriosis. Arch Gynecol Obstet 289, 1119–1124 (2014). https://doi.org/10.1007/s00404-013-3103-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-3103-6