Abstract
Introduction
Inhibins, dimeric peptide hormones composed of an α subunit and one of two possible β subunits (betaA or betaB), exhibit substantial roles in human reproduction and in endocrine-responsive tumors. Recently, two novel inhibin-beta subunits, defined as betaC and betaE, have been identified in humans. However, the prognostic significance and clinical implications of the novel inhibin-betaC subunit in endometrial cancers is still quite unclear.
Materials and methods
A series of 296 uterine endometrial carcinomas were immunohistochemically analyzed with specific antibody against the inhibin-betaC subunit. The staining reactions were correlated with several clinicopathological characteristics and the clinical outcome.
Results
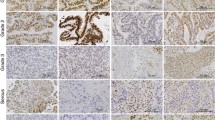
Endometrial cancer tissue demonstrated an immunolabelling against the inhibin-betaC subunit. The inhibin-betaC expression in endometrial carcinoma samples revealed a significant association with hemangiosis. However, the expression of this inhibin subunit did not affect patients’ progression-free, cause-specific and overall survival.
Conclusion
Overall, inhibin-betaC subunit was demonstrated in endometrial cancer tissue. This novel betaC subunit demonstrated a significant association with hemangiosis although without any impact on the patients’ survival. Moreover, the inhibin-betaC subunits did not constitute an independent prognostic parameter in endometrial cancer patients. Therefore, the isolated analysis of this subunit might be of minor prognostic value in identifying high-risk patients.


Similar content being viewed by others
References
Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I (2005) Endometrial cancer. Lancet 366(9484):491–505
Prat J (2004) Prognostic parameters of endometrial carcinoma. Hum Pathol 35(6):649–662
Jereczek-Fossa B, Badzio A, Jassem J (1999) Surgery followed by radiotherapy in endometrial cancer: analysis of survival and patterns of failure. Int J Gynecol Cancer 9(4):285–294
Sayin NC, Varol FG, Yuce MA, Kaplan P, Ahmet N, Sut N, Gucer F (2009) Do routine preoperative imaging techniques facilitate the operation in endometrial cancer? Arch Gynecol Obstet 280(2):211–215. doi:10.1007/s00404-008-0893-z
Lax SF (2004) Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch 444(3):213–223
Mylonas I, Worbs S, Shabani N, Kuhn C, Kunze S, Schulze S, Dian D, Gingelmaier A, Schindlbeck C, Bruning A, Sommer H, Jeschke U, Friese K (2009) Inhibin-alpha subunit is an independent prognostic parameter in human endometrial carcinomas: analysis of inhibin/activin-alpha, -betaA and -betaB subunits in 302 cases. Eur J Cancer 45(7):1304–1314. doi:S0959-8049(09)00002-1[pii]10.1016/j.ejca.2009.01.008
Worbs S, Shabani N, Mayr D, Gingelmaier A, Makrigiannakis A, Kuhn C, Jeschke U, Kupka MS, Friese K, Mylonas I (2007) Expression of the inhibin/activin subunits (-alpha, -betaA and -betaB) in normal and carcinogenic endometrial tissue: possible immunohistochemical differentiation markers. Oncol Rep 17(1):97–104
Xia Y, Schneyer AL (2009) The biology of activin: recent advances in structure, regulation and function. J Endocrinol 202(1):1–12. doi:JOE-08-0549[pii]10.1677/JOE-08-0549
Vale W, Wiater E, Gray P, Harrison C, Bilezikjian L, Choe S (2004) Activins and inhibins and their signaling. Ann N Y Acad Sci 1038:142–147
Risbridger GP, Ball EM, Wang H, Mellor SL, Peehl DM (2004) Re-evaluation of inhibin alpha subunit as a tumour suppressor in prostate cancer. Mol Cell Endocrinol 225(1–2):73–76
Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A (1992) Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360(6402):313–319
Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A (1994) Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci USA 91(19):8817–8821
Mylonas I, Makovitzky J, Richter DU, Jeschke U, Briese V, Friese K (2004) Expression of the inhibin-alpha subunit in normal, hyperplastic and malignant endometrial tissue: an immunohistochemical analysis. Gynecol Oncol 93(1):92–97
Hötten G, Neidhardt H, Schneider C, Pohl J (1995) Cloning of a new member of the TGF-beta family: a putative new activin beta C chain. Biochem Biophys Res Commun 206(2):608–613
Fang J, Yin W, Smiley E, Wang SQ, Bonadio J (1996) Molecular cloning of the mouse activin beta E subunit gene. Biochem Biophys Res Commun 228(3):669–674
Weissenbacher T, Bruning A, Kimmich T, Makovitzky J, Gingelmaier A, Mylonas I (2010) Immunohistochemical Labeling of the Inhibin/activin betaC subunit in normal human placental tissue and chorionic carcinoma cell lines. J Histochem Cytochem 58(8):751–757. doi:jhc.2010.956185[pii]10.1369/jhc.2010.956185
Mylonas I, Shabani N, Vogl J, Makovitzky J, Kunze S, Kuhn C, Schulze S, Friese K, Jeschke U (2007) Inhibin/activin subunits are immunohistochemically expressed in complete and partial hydatidiform moles. Anticancer Res 27(4A):1995–2000
Gingelmaier A, Bruning A, Kimmich T, Makovitzky J, Bergauer F, Schiessl B, Friese K, Mylonas I (2010) Inhibin/activin-betaE subunit is expressed in normal and pathological human placental tissue including chorionic carcinoma cell lines. Arch Gynecol Obstet. doi:10.1007/s00404-009-1340-5
Kimmich T, Bruning A, Kaufl SD, Makovitzky J, Kuhn C, Jeschke U, Friese K, Mylonas I (2010) Inhibin/activin-betaC and -betaE subunits in the Ishikawa human endometrial adenocarcinoma cell line. Arch Gynecol Obstet 282(2):185–191. doi:10.1007/s00404-009-1310-y
Käufl SD, Makovitzky J, Kuhn C, Kunze S, Jeschke U, Mylonas I (2010) Inhibin/activin-betaC subunit in human endometrial adenocarcinomas and HEC-1a adenocarcinoma cell line. In Vivo (in press)
Shabani N, Kuhn C, Kunze S, Schulze S, Mayr D, Dian D, Gingelmaier A, Schindlbeck C, Willgeroth F, Sommer H, Jeschke U, Friese K, Mylonas I (2007) Prognostic significance of oestrogen receptor alpha (ERalpha) and beta (ERbeta), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur J Cancer 43(16):2434–2444. doi:S0959-8049(07)00642-9[pii]10.1016/j.ejca.2007.08.014
Anonymous (1989) FIGO stages (announcements). Gynecol Oncol 35125–35127
Mylonas I, Jeschke U, Wiest I, Hoeing A, Vogl J, Shabani N, Kuhn C, Schulze S, Kupka MS, Friese K (2004) Inhibin/activin subunits alpha, beta-A and beta-B are differentially expressed in normal human endometrium throughout the menstrual cycle. Histochem Cell Biol 122(5):461–471
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53457–53481
Cox DR (1972) Regression models and life tables. J R Stat Soc B 34187–34220
Ehata S, Hanyu A, Fujime M, Katsuno Y, Fukunaga E, Goto K, Ishikawa Y, Nomura K, Yokoo H, Shimizu T, Ogata E, Miyazono K, Shimizu K, Imamura T (2007) Ki26894, a novel transforming growth factor-beta type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Cancer Sci 98(1):127–133
Ogino H, Yano S, Kakiuchi S, Muguruma H, Ikuta K, Hanibuchi M, Uehara H, Tsuchida K, Sugino H, Sone S (2008) Follistatin suppresses the production of experimental multiple-organ metastasis by small cell lung cancer cells in natural killer cell-depleted SCID mice. Clin Cancer Res 14(3):660–667
Tsuchida K, Nakatani M, Hitachi K, Uezumi A, Sunada Y, Ageta H, Inokuchi K (2009) Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal 7:15. doi:1478-811X-7-15[pii]10.1186/1478-811X-7-15
Mylonas I, Jeschke U, Winkler L, Makovitzky J, Richter DU, Briese V, Friese K (2003) Immunohistochemical expression of inhibin-alpha in human endometrium and the in vitro secretion of inhibin, estradiol and cortisol in cultured human endometrial glandular cells. Arch Gynecol Obstet 268(3):142–150. doi:10.1007/s00404-003-0526-5
Vogl J, Hoing A, Schulze S, Kuhn C, Wiest I, Shabani N, Jeschke U, Mylonas I, Friese K (2007) Expression of inhibins in the endometrial carcinoma cell line RL-95-2 after stimulation with cortisol and estradiol. Anticancer Res 27(4A):1989–1993
Mylonas I, Makovitzky J, Fernow A, Richter DU, Jeschke U, Briese V, Gerber B, Friese K (2005) Expression of the inhibin/activin subunits alpha (alpha), beta-A (betaA) and beta-B (betaB) in benign human endometrial polyps and tamoxifen-associated polyps. Arch Gynecol Obstet 272(1):59–66. doi:10.1007/s00404-004-0666-2
Risbridger GP, Schmitt JF, Robertson DM (2001) Activins and inhibins in endocrine and other tumors. Endocr Rev 22(6):836–858
Mylonas I (2010) Inhibin-alpha, -betaA and -betaB subunits in uterine non-endometrioid carcinomas: prognostic significance and clinical implications. Eur J Cancer. doi:S0959-8049(10)00476-4[pii]10.1016/j.ejca.2010.06.001
Sharifi N, Lechleider RJ, Farrar WL (2007) Transforming growth factor-beta receptor III downregulation in prostate cancer: is inhibin B a tumor suppressor in prostate? J Mol Endocrinol 39(5):329–332
Chabicovsky M, Herkner K, Rossmanith W (2003) Overexpression of activin beta(C) or activin beta(E) in the mouse liver inhibits regenerative deoxyribonucleic acid synthesis of hepatic cells. Endocrinology 144(8):3497–3504
Vejda S, Erlach N, Peter B, Drucker C, Rossmanith W, Pohl J, Schulte-Hermann R, Grusch M (2003) Expression of activins C and E induces apoptosis in human and rat hepatoma cells. Carcinogenesis 24(11):1801–1809
Wada W, Medina JJ, Kuwano H, Kojima I (2005) Comparison of the function of the beta(C) and beta(E) subunits of activin in AML12 hepatocytes. Endocr J 52(2):169–175
Gold EJ, Zhang X, Wheatley AM, Mellor SL, Cranfield M, Risbridger GP, Groome NP, Fleming JS (2005) betaA- and betaC-activin, follistatin, activin receptor mRNA and betaC-activin peptide expression during rat liver regeneration. J Mol Endocrinol 34(2):505–515
Lau AL, Kumar TR, Nishimori K, Bonadio J, Matzuk MM (2000) Activin betaC and betaE genes are not essential for mouse liver growth, differentiation, and regeneration. Mol Cell Biol 20(16):6127–6137
Gold E, Jetly N, O’Bryan MK, Meachem S, Srinivasan D, Behuria S, Sanchez-Partida LG, Woodruff T, Hedwards S, Wang H, McDougall H, Casey V, Niranjan B, Patella S, Risbridger G (2009) Activin C antagonizes activin A in vitro and overexpression leads to pathologies in vivo. Am J Pathol 174(1):184–195. doi:ajpath.2009.080296[pii]10.2353/ajpath.2009.080296
Mellor SL, Cranfield M, Ries R, Pedersen J, Cancilla B, de Kretser D, Groome NP, Mason AJ, Risbridger GP (2000) Localization of activin beta(A)-, beta(B)-, and beta(C)-subunits in humanprostate and evidence for formation of new activin heterodimers of beta(C)-subunit. J Clin Endocrinol Metab 85(12):4851–4858
Mellor SL, Ball EM, O’Connor AE, Ethier JF, Cranfield M, Schmitt JF, Phillips DJ, Groome NP, Risbridger GP (2003) Activin betaC-subunit heterodimers provide a new mechanism of regulating activin levels in the prostate. Endocrinology 144(10):4410–4419
Razanajaona D, Joguet S, Ay AS, Treilleux I, Goddard-Leon S, Bartholin L, Rimokh R (2007) Silencing of FLRG, an antagonist of activin, inhibits human breast tumor cell growth. Cancer Res 67(15):7223–7229
Ramachandran A, Marshall ES, Love DR, Baguley BC, Shelling AN (2009) Activin is a potent growth suppressor of epithelial ovarian cancer cells. Cancer Lett 285(2):157–165. doi:S0304-3835(09)00344-9[pii]10.1016/j.canlet.2009.05.010
Katik I, Mackenzie-Kludas C, Nicholls C, Jiang FX, Zhou S, Li H, Liu JP (2009) Activin inhibits telomerase activity in cancer. Biochem Biophys Res Commun 389(4):668–672. doi:S0006-291X(09)01857-9[pii]10.1016/j.bbrc.2009.09.055
Di Simone N, Schneyer AL, Caliandro D, Castellani R, Caruso A (2002) Regulation of endometrial adenocarcinoma cell proliferation by Activin-A and its modulation by 17beta-estradiol. Mol Cell Endocrinol 192(1–2):187–195
Acknowledgments
We would like to thank Dr. S. Worbs, Mrs. S. Schulze, Dr. D. Dian, Dr. A. Gingelmaier, Dr. C. Schindlbeck, Prof. H. Sommer and Prof. Dr. U. Jeschke for their help in conducting the primary study regarding inhibin-α, -βA and -βB expression in endometrial cancer. Additionally we would like to thank Prof. D. Hölzel, Institute of Medical Informatics, Biometry and Epidemiology, Ludwig-Maximilians-University Munich and Mr. M. Schmidt of the Munich Tumor Registry for supplying the survival data. This study was partially supported by the FöFoLe program of the Ludwig-Maximilians-University Munich (297/03), the Friedrich-Baur-Institute Munich and the Weigland Stipendium Program of the Ludwig-Maximilians-University Munich for I. Mylonas.
Conflict of interest
The authors declare that they have no competing interests. The authors have full control of all primary data and agree to allow the journal to review their data if required.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Käufl, S.D., Kuhn, C., Kunze, S. et al. Inhibin/activin-betaC subunit does not represent a prognostic parameter in human endometrial cancer. Arch Gynecol Obstet 284, 199–207 (2011). https://doi.org/10.1007/s00404-010-1614-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1614-y