Abstract
Purpose
The production of epithelial neutrophil activating peptide-78 (NA-78) and the interleukins IL-8 and IL-6 by endometrial stromal cells is stimulated by pro-inflammatory interleukin-1 (IL-1) and tumour necrosis factor-α (TNF-α). IL-8 is suggested to play a role in the pathogenesis of endometriosis, and in these women the peritoneal fluid concentrations of ENA-78 and IL-8 are increased. TNF-α has been tested together with interferon-γ because of their cooperative stimulation of IL-6. The release of IL-8, however, is inhibited with increasing interferon levels. The aim of the study was the analysis of the production of ENA-78, IL-6 and IL-8 by cultured human endometrial stromal cells in the presence of varying concentrations of IL-1β, TNF-α, and interferon-γ.
Methods
Eutopic endometrial tissue was obtained from seven cycling, endometriosis-free women undergoing laparoscopy for reasons of infertility or pain. The release of ENA-78, IL-8 and IL-6 by the isolated and monolayer cultured stromal cell fraction in the presence of IL-1β (0.08 to 50 ng/mL), TNF-α, and interferon-γ (both 20 to 500 ng/mL) was determined.
Results
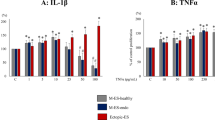
IL-1β stimulated the production of IL-8, IL-6, and ENA-78 dose dependently from 0.08 to 2.0 ng/mL (ENA-78) or to 10 ng/mL (IL-8, IL-6); at 50 ng/mL a decrease in release was observed for IL-8 and IL-6. TNF-α stimulation yielded a plateau between 20 and 100 ng/mL. Interferon-γ stimulated IL-6 and inhibited IL-8 production above 20 ng/mL. ENA-78 release was largely unaffected by interferon-γ.
Conclusions
IL-1β and TNF-α stimulate stromal cytokine production cumulatively with different dose–response curves. The presence of interferon-γ has opposite effects on IL-8 and IL-6. TNF-α and interferon-γ should be investigated separately in future in vitro studies with endometrial cells and explants.



Similar content being viewed by others
References
Koch AE, Volin MV, Woods JM et al (2001) Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and epithelial neutrophil activating peptide 78 in the rheumatoid joint. Arthritis Rheumatol 44:31–40
Walz A, Schmutz P, Mueller C, Schnyder S (1997) Regulation and function of CXC chemokine ENA-78 in monocytes and its role in disease. J Leukoc Biol 62:604–611
Nasu K, Arima K, Kai K, Fujisawa K, Nishida M, Miyakawa I (2001) Expression of epithelial neutrophil-activating peptide 78 in cultured human endometrial stromal cells. Mol Hum Reprod 7:453–458
Persson T, Monsef N, Andersson P et al (2003) Expression of the neutrophil-activating CXC chemokine ENA-78/CXCL5 by human eosinophils. Clin Exp Allergy 33:531–537
Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG (1996) Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril 65:299–304
Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN (1995) Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril 63:929–932
Iwabe T, Harada T, Tsudo T, Tanikawa M, Onohara Y, Terakawa N (1998) Pathogenetic significance of increased levels of interleukin-8 in the peritoneal fluid of patients with endometriosis. Fertil Steril 69:924–930
Harada T, Enatsu A, Mitsunari M, Nagano Y, Ito M, Tsudo T, Taniguchi F, Iwabe T, Tanikawa M, Terakawa N (1999) Role of cytokines in progression of endometriosis. Gynecol Obstet Invest 47(Suppl 1):34–39
Mueller MD, Mazzucchelli L, Buri C, Lebovic DI, Dreher E, Taylor RN (2003) Epithelial neutrophil-activating peptide 78 concentrations are elevated in the peritoneal fluid of women with endometriosis. Fertil Steril 79(S1):815–820
Suzumori N, Katano K, Suzumori K (2004) Peritoneal fluid concentrations of epithelial neutrophil-activating peptide-78 correlated with the severity of endometriosis. Fertil Steril 81:305–308
Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R (1996) Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol 174:1522–1526
Harada T, Yoshioka H, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, Terakawa N (1997) Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol 176:593–597
Mahnke JL, Dawood MY, Huang JC (2000) Vascular endothelial growth factor and interleukin-6 in peritoneal fluid of women with endometriosis. Fertil Steril 73:166–170
Bersinger NA, von Roten S, Wunder DM, Raio L, Dreher E, Mueller MD (2006) PAPP-A and osteoprotegerin, together with interleukin-8 and RANTES, are elevated in the peritoneal fluid of women with endometriosis. Am J Obstet Gynecol 195:103–108
Bersinger NA, Frischknecht F, Taylor RN, Mueller MD (2008) Basal and cytokine-stimulated production of epithelial neutrophil activating peptide-78 (ENA-78) and interleukin-8 (IL-8) by cultured human endometrial epithelial and stromal cells. Fertil Steril 89:1530–1536
Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M, Terakawa N (2000) Tumour necrosis factor-α promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endorinol Metab 85:824–829
Nasu K, Matsui N, Narahara H, Tanaka Y, Miyakawa I (1998) Effects of interferon-γ on cytokine production by endometrial stromal cells. Hum Reprod 13:2598–2601
Tabibzadeh SS, Satyaswaroop PG, Rao PN (1988) Antiproliferative effect of interferon-gamma in human endometrial epithelial cells in vitro: potential local growth modulatory role in endomtrium. J Clin Endocrinol Metab 67:131–138
Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA (2009) Interferon-gamma in successful pregnancies. Biol Reprod 80:848–859
Ryan IP, Schriock ED, Taylor RN (1994) Isolation, characterisation, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 78:642–649
Wunder DM, Mueller MD, Birkhäuser MH, Bersinger NA (2006) Increased ENA-78 in the follicular fluid of patients with endometriosis. Acta Obstet Gynecol Scand 85:336–342
Tabibzadeh SS, Sun XZ (1992) Cytokine expression in human endometrium throughout the menstrual cycle. Hum Reprod 7:1214–1221
Arici A, Seli E, Senturk LM, Gutierrez LS, Oral E, Taylor HS (1998) Interleukin-8 in the human endometrium. J Clin Endocrinol Metab 83:1783–1787
Vandermolen DT, Gu Y (1996) Human endometrial interleukin-6 (IL-6): in vivo messenger ribonucleic acid expression, in vitro protein production, and stimulation thereof by IL-1β. Fertil Steril 66:741–747
Von Wolff M, Stieger S, Lumpp K, Bucking J, Strowitzki T, Thaler CJ (2002) Endometrial interleukin-6 in vitro is not regulated directly by female steroid hormones, but by pro-inflammatory cytokines and hypoxia. Mol Hum Reprod 8:1096–1102
Arici A, Head JR, McDonald PC, Casey ML (1993) Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol 94:195–204
Nasu K, Matsui N, Narahara H et al (1998) MaMi, a human endometrial stromal sarcoma cell line that constitutively produces interleukin-6, interleukin-8, and monocyte chemoattractant protein-1. Arch Pathol Lab Med 122:836–841
Barker JN, Sarma V, Mitra RS, Dixit VM, Nickoloff BJ (1990) Marked synergism between tumour necrosis factor-alpha and interferon-gamma in regulation of keratinocyte-derived adhesion molecules and chemotactic factors. J Clin Invest 85:605–608
Chegini N, Luo X, Pan Q, Rhoton A, Archer DF (2007) Endometrial expression of epithelial neutrophil-activating peptide-78 during the menstrual cycle or in progestin-only contraceptive users with breakthrough bleeding and the influence of doxycycline therapy. Hum Reprod 22:427–433
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bersinger, N.A., Günthert, A.R., McKinnon, B. et al. Dose–response effect of interleukin (IL)-1β, tumour necrosis factor (TNF)-α, and interferon-γ on the in vitro production of epithelial neutrophil activating peptide-78 (ENA-78), IL-8, and IL-6 by human endometrial stromal cells. Arch Gynecol Obstet 283, 1291–1296 (2011). https://doi.org/10.1007/s00404-010-1520-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1520-3