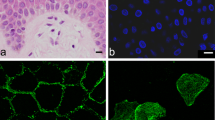
Abstract Proteolytic enzymes play crucial roles in the formation of the stratum corneum barrier tissue and in its subsequent maturation. Despite this, the proteases involved in stratum corneum physiology are not well characterized. Hence, studies were performed to identify these proteolytic enzymes present in the peripheral layers of this tissue using a combination of tape stripping and zymography. Using this approach, a novel human cysteine protease was identified and characterized, and named stratum corneum thiol protease (SCTP). Gelatin zymography revealed that SCTP is composed of two variants with apparent molecular weights of 34 and 35 kDa which do not correspond to any previously described stratum corneum protease. Mechanistically SCTP belongs to the cysteine proteinase class as shown by: (1) acid protease activity, (2) a requirement for mild reducing conditions, and (3) the specific inhibition of activity by E64 and Z-phe-ala-diazomethylketone. Further analysis using concanavalin A affinity chromatography demonstrated that the two 34 and 35 kDa variants are both glycoproteins, which, after removal of the oligosaccharide sidechains with the specific enzyme N-glycopeptidase F, reveal a single active core protease of 32 kDa. SCTP did not crossreact with antibodies raised against the lysosomal cysteine proteases cathepsins B, H or L, thereby distinguishing it from the classical cysteine cathepsins. Localization studies revealed that SCTP is present at all depths in the stratum corneum, thereby precluding microbial contamination as the enzyme source. Moreover, it was also present at all body sites investigated, except for the hyperkeratotic palmoplantar stratum corneum. SCTP was found to be a product of late differentiation in cultured human keratinocytes; the enzyme was synthesized by differentiated calcium-switched cells and secreted into the medium, whereas nondifferentiated basal keratinocytes did not produce this protease. Moreover, human fibroblast cultures did not produce the enzyme, suggesting that SCTP is not produced by the dermis and hence is epidermal specific. The function of SCTP is unknown, but the observed gelatinolytic activity coupled with its secretion into the medium by cultured keratinocytes indicates that physiologically it is responsible for the degradation of extracellular structural proteins. Furthermore, the optimal activity at acid pH suggests that it can function in the acidic environment of the stratum corneum. It remains to be elucidated whether this enzyme has a role in desquamation.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 14 August 1998 / Received after revision: 23 November 1998 / Accepted: 26 November 1998
Rights and permissions
About this article
Cite this article
Watkinson, A. Stratum corneum thiol protease (SCTP): a novel cysteine protease of late epidermal differentiation. Arch Dermatol Res 291, 260–268 (1999). https://doi.org/10.1007/s004030050406
Issue Date:
DOI: https://doi.org/10.1007/s004030050406