Abstract
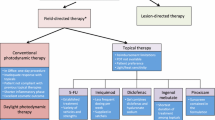
Cutaneous field cancerization in dermatology describes the anatomic region of photodamaged skin with actinic keratoses (AKs) or cutaneous squamous cell carcinoma (cSCC) that is surrounded by cellular atypia, forming a dysplastic field. The concept of field cancerization is especially relevant in dermatology, as actinic keratoses and the surrounding dysplastic region can progress to carcinomas, necessitating the treatment of the field. Recent research has focused on field-directed therapy using topical agents. This study aims to systematically review randomized controlled trials on topical treatments for actinic keratosis field cancerization, following the PRISMA guidelines. Clinical recommendations were based on the Oxford Centre for Evidence-Based Medicine. We identified 20 original randomized controlled trials for topical cutaneous field therapy. 0.5% 5-Fluorouracil/salicylic acid and 0.5% 5-fluorouracil received a clinical recommendation grade of A, while diclofenac sodium received a clinical recommendation grade of B. Calcipotriol/5-fluorouracil, Imiquimod, sunscreen combination therapies, and tirbanibulin received a recommendation grade of C. This review provides a framework for clinicians when considering topical treatments for patients with field cancerization.

Similar content being viewed by others
Data availability
The data that support the evidence presented in this article is accessible from public databases including PubMed, Embase, and Cochrane.
References
Willenbrink TJ et al (2020) Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol 83(3):709–717
Huang A et al (2019) Updates on treatment approaches for cutaneous field cancerization. Curr Dermatol Rep 8(3):122–132
Lanoue J, Chen C, Goldenberg G (2016) Actinic keratosis as a marker of field cancerization in excision specimens of cutaneous malignancies. Cutis 97(6):415–420
Christensen SR (2018) Recent advances in field cancerization and management of multiple cutaneous squamous cell carcinomas. F1000Res. https://doi.org/10.12688/f1000research.12837.1
Rogers HW et al (2015) Incidence estimate of nonmelanoma skin cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol 151(10):1081–1086
Figueras Nart I et al (2018) Defining the actinic keratosis field: a literature review and discussion. J Eur Acad Dermatol Venereol 32(4):544–563
Torezan LA, Festa-Neto C (2013) Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol 88(5):775–786
Cheraghlou S, Feng H, Cohen JM (2020) Trends in the access and cost of photodynamic therapy among Medicare beneficiaries in the United States, 2012–2017. JAMA Dermatol 156(9):1021–1022
Jansen MHE et al (2020) A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br J Dermatol 183(4):738–744
Nelson CG (2011) Diclofenac gel in the treatment of actinic keratoses. Ther Clin Risk Manag 7:207–211
Korman N et al (2005) Dosing with 5% imiquimod cream 3 times per week for the treatment of actinic keratosis: results of two phase 3, randomized, double-blind, parallel-group, vehicle-controlled trials. Arch Dermatol 141(4):467–473
Heron CE, Feldman SR (2021) Ingenol mebutate and the treatment of actinic keratosis. J Drugs Dermatol 20(1):102–104
Rosso JD et al (2021) Advances and considerations in the management of actinic keratosis: an expert consensus panel report. J Drugs Dermatol 20(8):888–893
Jansen MHE et al (2019) Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med 380(10):935–946
Oxford Centre for Evidence-Based Medicine: levels of evidence (March 2009). Centre for Evidence Based Medicine 2009. cited 7 Aug 2023.
Ghafouri-Fard S et al (2021) 5-Fluorouracil: a narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. Front Oncol 11:658636
Stockfleth E et al (2017) Efficacy and safety of 5-fluorouracil 0.5%/salicylic acid 10% in the field-directed treatment of actinic keratosis: a phase III, randomized, double-blind, vehicle-controlled trial. Dermatol Ther (Heidelb) 7(1):81–96
Stockfleth E et al (2011) Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol 165(5):1101–1108
Jorizzo J et al (2002) Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis 70(6):335–339
Loven K et al (2002) Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin Ther 24(6):990–1000
Wolf JE Jr et al (2001) Topical 3.0% diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol 40(11):709–713
Mazzella C et al (2018) Management of clinical and subclinical actinic keratoses with histological and immunohistochemical assessments by confocal microscopy. Dermatol Ther 31(5):e12672
Ulrich M et al (2010) Reflectance confocal microscopy for noninvasive monitoring of therapy and detection of subclinical actinic keratoses. Dermatology 220(1):15–24
Rivers JK et al (2002) Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol 146(1):94–100
Kim GK (2010) The rationale behind topical vitamin d analogs in the treatment of psoriasis: where does topical calcitriol fit in? J Clin Aesthet Dermatol 3(8):46–53
Cunningham TJ et al (2017) Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest 127(1):106–116
Bubna AK (2015) Imiquimod—its role in the treatment of cutaneous malignancies. Indian J Pharmacol 47(4):354–359
Ulrich C et al (2007) Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo-controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br J Dermatol 157(Suppl 2):25–31
Chen K et al (2003) Short-course therapy with imiquimod 5% cream for solar keratoses: a randomized controlled trial. Australas J Dermatol 44(4):250–255
Swanson N et al (2010) Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol 62(4):582–590
Jorizzo J et al (2007) Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol 57(2):265–268
Alomar A, Bichel J, McRae S (2007) Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol 157(1):133–141
Szeimies RM et al (2004) Imiquimod 5% cream for the treatment of actinic keratosis: results from a phase III, randomized, double-blind, vehicle-controlled, clinical trial with histology. J Am Acad Dermatol 51(4):547–555
Gebauer K, Shumack S, Cowen PS (2009) Effect of dosing frequency on the safety and efficacy of imiquimod 5% cream for treatment of actinic keratosis on the forearms and hands: a phase II, randomized placebo-controlled trial. Br J Dermatol 161(4):897–903
Carducci M et al (2015) Comparative effects of sunscreens alone vs sunscreens plus DNA repair enzymes in patients with actinic keratosis: clinical and molecular findings from a 6-month, randomized. Clinical Study J Drugs Dermatol 14(9):986–990
Bobyr I et al (2019) Fluorescent photodiagnostic evaluation of field cancerization treated with a medical device containing piroxicam 08% and sunscreen SPF 50+ for actinic keratosis. Photodermatol Photoimmunol Photomed 35(4):277–279
Alvares BA et al (2022) Efficacy of sunscreen with photolyase or regular sunscreen associated with topical antioxidants in treating advanced photodamage and cutaneous field cancerization: a randomized clinical trial. An Bras Dermatol 97(2):157–165
Luze H et al (2020) DNA repair enzymes in sunscreens and their impact on photoageing-A systematic review. Photodermatol Photoimmunol Photomed 36(6):424–432
Berman B, Grada A, Berman DK (2022) Profile of tirbanibulin for the treatment of actinic keratosis. J Clin Aesthet Dermatol 15(10 Suppl 1):S3–S10
Blauvelt A et al (2021) Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med 384(6):512–520
Kishi P, Price CJ (2018) Life-threatening reaction with topical 5-fluorouracil. Drug Saf Case Rep 5(1):4
Johnson MR et al (1999) Life-threatening toxicity in a dihydropyrimidine dehydrogenase-deficient patient after treatment with topical 5-fluorouracil. Clin Cancer Res 5(8):2006–2011
Papanastasopoulos P, Stebbing J (2014) Molecular basis of 5-fluorouracil-related toxicity: lessons from clinical practice. Anticancer Res 34(4):1531–1535
Suh SA-O et al (2020) The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol 59:1365–4632
Ortonne JP et al (2010) Effectiveness of cross polarized light and fluorescence diagnosis for detection of sub-clinical and clinical actinic keratosis during imiquimod treatment. Exp Dermatol 19(7):641–647
Baker C et al (2022) Method of Assessing Skin Cancerization and Keratoses(TM) (MASCK): development and photographic validation in multiple anatomical sites of a novel assessment tool intended for clinical evaluation of patients with extensive skin field cancerization. Clin Exp Dermatol 47(6):1144–1153
Acknowledgements
PP performed the literature search and wrote the main manuscript. PP and JW reviewed the studies independently. JW, DB, JM, and EA helped to review the manuscript. JJ helped to develop the concept and edit/review the final manuscript. All authors reviewed and approved the final manuscript.
Funding
No funding has been received for this article. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the U.S. Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Contributions
PP performed the literature search and wrote the main manuscript. PP and JW reviewed the studies independently. JW, DB, JM, and EA helped to review the manuscript. JJ helped to develop the concept and edit/review the final manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, P., Wang, J., Bitterman, D. et al. Systematic review of randomized controlled trials of topicals for actinic keratosis field therapy. Arch Dermatol Res 316, 108 (2024). https://doi.org/10.1007/s00403-024-02839-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-02839-y