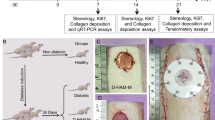
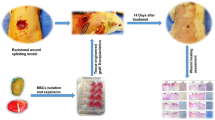
Abstract
The occurrence of wounds and defects in the healing process is one of the main challenges in diabetic patients. Herein, we investigated whether adipose-derived stem cells (ADSCs)-derived exosomes loaded bioengineered micro-porous three-dimensional amniotic membrane-scaffold (AMS) could promote healing in diabetic rats. Sixty diabetic rats were randomly allocated into the control group, exosome group, AMS group, and AMS + Exo group. On days 7, 14, and 21, five rats from each group were sampled for stereological, immunohistochemical, molecular, and tensiometrical assessments. Our results indicated that the wound closure rate, the total volumes of newly formed epidermis and dermis, the numerical densities of fibroblasts and proliferating cells, the length density blood vessels, collagen density as well as tensiometrical parameters of the healed wounds were considerably greater in the treated groups than in the control group, and these changes were more obvious in the AMS + Exo ones. Furthermore, the expression of TGF-β, bFGF, and VEGF genes was meaningfully upregulated in all treated groups compared to the control group and were greater in the AMS + Exo group. This is while expression of TNF-α and IL-1β, as well as cell numerical densities of neutrophils, M1 macrophages, and mast cells decreased more considerably in the AMS + Exo group in comparison with the other groups. Generally, it was found that using both AMS transplantation and ADSCs-derived exosomes has more effect on diabetic wound healing.











Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, Gu L, Zhang C, Wang B, Wei W (2021) Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif 54:e12993. https://doi.org/10.1111/cpr.12993
Bagheri M, Amini A, Abdollahifar M-A, Ghoreishi SK, Piryaei A, Pouriran R, Chien S, Dadras S, Rezaei F, Bayat M (2018) Effects of photobiomodulation on degranulation and number of mast cells and wound strength in skin wound healing of streptozotocin-induced diabetic rats. Photomed Laser Surg 36:415–423. https://doi.org/10.1089/pho.2018.4453
Barnes CA, Brison J, Michel R, Brown BN, Castner DG, Badylak SF, Ratner BD (2011) The surface molecular functionality of decellularized extracellular matrices. Biomaterials 32:137–143. https://doi.org/10.1016/j.biomaterials.2010.1009.1007
Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Álvarez V, Tarazona R, Casado JG (2014) Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol 5:556. https://doi.org/10.3389/fimmu.2014.00556
Bowers S, Franco E (2020) Chronic wounds: evaluation and management. Am Fam Physician 101:159–166
Castellanos G, Bernabe-Garcia A, Moraleda JM, Nicolas FJ (2017) Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta 59:146–153. https://doi.org/10.1016/j.placenta.2017.1004.1005
Chang C-L, Sung P-H, Chen K-H, Shao P-L, Yang C-C, Cheng B-C, Lin K-C, Chen C-H, Chai H-T, Chang H-W (2018) Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am J Transl Res 10:1053
Cheshmi H, Mohammadi H, Akbari M, Nasiry D, Rezapour-Nasrabad R, Bagheri M, Abouhamzeh B, Poorhassan M, Mirhoseini M, Mokhtari H (2023) Human placental mesenchymal stem cell-derived exosomes in combination with hyperbaric oxygen synergistically promote recovery after spinal cord injury in rats. Neurotox Res. https://doi.org/10.1007/s12640-12023-00649-12640
Costa A, Naranjo JD, Londono R, Badylak SF (2017) Biologic scaffolds. Cold Spring Harb Perspect Med 7:a025676. https://doi.org/10.1101/cshperspect.a025676
Davoodi S, Ebrahimpour-Malekshah R, Ayna Ö, Akbari M, Raoofi A, Mokhtari H, Izanlu M, Modanloo F, Nasiry D (2022) Decellularized human amniotic membrane engraftment in combination with adipose-derived stem cells transplantation, synergistically improved diabetic wound healing. J Cosmet Dermatol. https://doi.org/10.1111/jocd.15394
Ebrahimpour-Malekshah R, Amini A, Zare F, Mostafavinia A, Davoody S, Deravi N, Rahmanian M, Hashemi SM, Habibi M, Ghoreishi SK (2020) Combined therapy of photobiomodulation and adipose-derived stem cells synergistically improve healing in an ischemic, infected and delayed healing wound model in rats with type 1 diabetes mellitus. BMJ Open Diabetes Res Care 8:e001033. https://doi.org/10.1136/bmjdrc-002019-001033
Enoch S, Leaper DJ (2008) Basic science of wound healing. Surg Infect (Larchmt) 26:31–37. https://doi.org/10.1016/j.mpsur.2007.1011.1005
Falanga V, Isseroff RR, Soulika AM, Romanelli M, Margolis D, Kapp S, Granick M, Harding K (2022) Chronic wounds. Nat Rev Dis Primers 8:50. https://doi.org/10.1038/s41572-41022-00377-41573
Gould LJ, Leong M, Sonstein J, Wilson S (2005) Optimization and validation of an ischemic wound model. Wound Repair Regen 13:576–582. https://doi.org/10.1111/j.1524-1475X.2005.00080.x
Han G, Ceilley R (2017) Chronic wound healing: a review of current management and treatments. Adv Ther 34:599–610. https://doi.org/10.1007/s12325-12017-10478-y
Higa K, Shimmura S, Shimazaki J, Tsubota K (2005) Hyaluronic acid-CD44 interaction mediates the adhesion of lymphocytes by amniotic membrane stroma. Cornea 24:206–212. https://doi.org/10.1097/1001.ico.0000133999.0000145262.0000133983
Hong P, Yang H, Wu Y, Li K, Tang Z (2019) The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther 10:1–12. https://doi.org/10.1186/s13287-13019-11358-y
Howard V, Reed M (2004) Unbiased stereology: three-dimensional measurement in microscopy. Garland Science, New York
Hsiao Y-C, Lee H-W, Chen Y-T, Young T-H, Yang T-L (2011) The impact of compositional topography of amniotic membrane scaffold on tissue morphogenesis of salivary gland. Biomaterials 32:4424–4432. https://doi.org/10.1016/j.biomaterials.2011.4402.4057
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L (2016) Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep 6:32993. https://doi.org/10.1038/srep32993
Intini C, Elviri L, Cabral J, Mros S, Bergonzi C, Bianchera A, Flammini L, Govoni P, Barocelli E, Bettini R (2018) 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr Polym 199:593–602. https://doi.org/10.1016/j.carbpol.2018.1007.1057
Izadi K, Ganchi P (2005) Chronic wounds. Clin Plast Surg 32:209–222. https://doi.org/10.1016/j.cps.2004.1011.1011
Izanlu M, Khalatbary A, Aliabadi A, Davoodi S, Raoofi A, Modanloo F, Nasiry D (2022) Synergistic effect of hyperbaric oxygen and decellularized human amniotic membrane on full-thickness diabetic wound healing in rats. J Maz Univ Med 32:1–15
Karuri NW, Liliensiek S, Teixeira AI, Abrams G, Campbell S, Nealey PF, Murphy CJ (2004) Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci 117:3153–3164. https://doi.org/10.1242/jcs.01146
Koizumi N, Inatomi T, Sotozono C, Fullwood NJ, Quantock AJ, Kinoshita S (2000) Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20:173–177. https://doi.org/10.1089/ten.tec.2013.0298
Li D, Wu N (2022) Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res Clin Pract. https://doi.org/10.1016/j.diabres.102022.109882
Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M (2018) Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med 50:1–14. https://doi.org/10.1038/s12276-12018-10058-12275
Mamede AC, Carvalho M, Abrantes AM, Laranjo M, Maia C, Botelho M (2012) Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 349:447–458. https://doi.org/10.1007/s00441-00012-01424-00446
Martin P, Nunan R (2015) Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 173:370–378. https://doi.org/10.1111/bjd.13954
Milan PB, Lotfibakhshaiesh N, Joghataie M, Ai J, Pazouki A, Kaplan D, Kargozar S, Amini N, Hamblin M, Mozafari M (2016) Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater 45:234–246. https://doi.org/10.1016/j.actbio.2016.1008.1053
Mohammadi AA, Eskandari S, Johari HG, Ao R (2017) Using amniotic membrane as a novel method to reduce post-burn hypertrophic scar formation: a prospective follow-up study. J Cutan Aesthet Surg 10:13–17. https://doi.org/10.4103/JCAS.JCAS_4109_4116
Mokoena D, Kumar SSD, Houreld NN, Abrahamse H (2018) Role of photobiomodulation on the activation of the Smad pathway via TGF-β in wound healing. J Photochem Photobiol B, Biol 189:138–144. https://doi.org/10.1016/j.jphotobiol.2018.1010.1011
Murphy SV, Skardal A, Song L, Sutton K, Haug R, Mack DL, Jackson J, Soker S, Atala A (2017) Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Transl Med 6:2020–2032. https://doi.org/10.1002/sctm.2017-0053
Nasiry D, Khalatbary AR (2023) Stem cell-derived extracellular vesicle-based therapy for nerve injury: a review of the molecular mechanisms. World Neurosurg. https://doi.org/10.1016/j.wnsx.102023.100201
Nasiry D, Khalatbary AR, Abdollahifar M-A, Amini A, Bayat M, Noori A, Piryaei A (2020) Engraftment of bioengineered three-dimensional scaffold from human amniotic membrane-derived extracellular matrix accelerates ischemic diabetic wound healing. Arch Dermatol Res. https://doi.org/10.1007/s00403-00020-02137-00403
Nasiry D, Khalatbary AR, Abdollahifar M-A, Bayat M, Amini A, Ashtiani MK, Rajabi S, Noori A, Piryaei A (2022) SDF-1α loaded bioengineered human amniotic membrane-derived scaffold transplantation in combination with hyperbaric oxygen improved diabetic wound healing. J Biosci Bioeng. https://doi.org/10.1016/j.jbiosc.2022.1001.1012
Nasiry D, Khalatbary AR, Ghaemi A, Ebrahimzadeh MA, Hosseinzadeh MH (2022) Topical administration of Juglans regia L. leaf extract accelerates diabetic wound healing. BMC Complement Med Ther 22:1–12. https://doi.org/10.1186/s12906-12022-03735-12906
Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM (2008) Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater 15:88–99. https://doi.org/10.2203/ecm.v22015a22207
Raoofi A, Delbari A, Nasiry D, Eslampour H, Golmohammadi R, sadat Javadinia S, Sadrzadeh R, Mojadadi M-S, Rustamzadeh A, Akhlaghi M, (2022) Caffeine modulates apoptosis, oxidative stress, and inflammation damage induced by tramadol in cerebellum of male rats. J Chem Neuroanat 123:1–10. https://doi.org/10.1016/j.jchemneu.2022.102116
Raoofi A, Rezaie MJ, Delbari A, Ghoreishi SA-H, Sichani PH, Maleki S, Nasiry D, Akhlaghi M, Ebrahimi V, Khaneghah AM (2022) Therapeutic potentials of the caffeine in polycystic ovary syndrome in a rat model: Via modulation of proinflammatory cytokines and antioxidant activity. Allergol Immunopathol 50:137–146. https://doi.org/10.1586/aei.v15550i15586.15715
Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A (2021) Immunology of acute and chronic wound healing. Biomolecules 11:700. https://doi.org/10.3390/biom11050700
Ryzhuk V, Zeng X, Wang X, Melnychuk V, Lankford L, Farmer D, Wang A (2018) Human amnion extracellular matrix derived bioactive hydrogel for cell delivery and tissue engineering. Mater Sci Eng C Mater Biol Appl 85:191–202. https://doi.org/10.1016/j.msec.2017.1012.1026
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.102019.107843
Seo Y, Kim H-S, Hong I-S (2019) Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int 2019:5126156. https://doi.org/10.1155/5122019/5126156
Seyed Sharifi SH, Nasiry D, Mahmoudi F, Etezadpour M, Ebrahimzadeh MA (2021) Evaluation of sambucus ebulus fruit extract in full-thickness diabetic wound healing in rats. J Maz Univ Med 31:11–25
Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Badiavas EV (2015) Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 24:1635–1647. https://doi.org/10.1089/scd.2014.0316
Siqueira MF, Li J, Chehab L, Desta T, Chino T, Krothpali N, Behl Y, Alikhani M, Yang J (2010) Impaired wound healing in mouse models of diabetes is mediated by TNF-a dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetologia 53:378–388. https://doi.org/10.1007/s00125-00009-01529-y
Wang L, Hu L, Zhou X, Xiong Z, Zhang C, Shehada HM, Hu B, Song J, Chen L (2017) Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep 7:13321. https://doi.org/10.1038/s41598-13017-12919-x
Wang S, Olson EN (2009) AngiomiRs—key regulators of angiogenesis. Curr Opin Genet Dev 19:205–211. https://doi.org/10.1016/j.gde.2009.1004.1002
Xiao S, Xiao C, Miao Y, Wang J, Chen R, Fan Z, Hu Z (2021) Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther 12:255. https://doi.org/10.1186/s13287-13021-02333-13286
Yang W-Z, Yang J, Xue L-P, Xiao L-B, Li Y (2017) MiR-126 overexpression inhibits high glucose-induced migration and tube formation of rhesus macaque choroid-retinal endothelial cells by obstructing VEGFA and PIK3R2. J Diabetes Complicat 31:653–663. https://doi.org/10.1016/j.jdiacomp.2016.1012.1004
Yu F, Witman N, Yan D, Zhang S, Zhou M, Yan Y, Yao Q, Ding F, Yan B, Wang H (2020) Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model. Stem Cell Res Ther 11:1–20. https://doi.org/10.1186/s13287-13020-02008-13288
Yu H, Chen X, Cai J, Ye D, Wu Y, Fan L, Liu P (2019) Novel porous three-dimensional nanofibrous scaffolds for accelerating wound healing. Chem Eng 369:253–262. https://doi.org/10.1016/j.cej.2019.1003.1091
Zhang W, Bai X, Zhao B, Li Y, Zhang Y, Li Z, Wang X, Luo L, Han F, Zhang J (2018) Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res 370:333–342. https://doi.org/10.1016/j.yexcr.2018.1006.1035
Zhu L, Huang X, Yu W, Chen H, Chen Y, Dai Y (2018) Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 50:e12871. https://doi.org/10.1111/and.12871
Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z (2008) Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem 26:664–675. https://doi.org/10.1002/cbf.1488
Acknowledgements
We would like to express our appreciation to Prof. Abbas Piryaei for his cooperation and technical support.
Funding
The current project was financially supported by grants to D. N. from Mazandaran University of Medical Sciences (Grant No.13758), Sari, Iran.
Author information
Authors and Affiliations
Contributions
A.R.K. contributed to the study design, data acquisition, and analysis, as well as drafting of the manuscript. M.O. contributed to the study design and fabrication of AMS and characterization. M.P. and M.As. contributed to isolation and characterization of ADSCs-derived exosomes. S.T. designed molecular assessments and analyses. R.E-M. designed tensiometrical tests, and contributed to acquisition and analysis of the data. D.N. supervised the study, provided financial support, and contributed to the study concept and design, interpretation of data, and editing and final approval of the manuscript. M.Ak. provided financial support, interpretation of data, and editing and final approval of the manuscript. A.R. designed stereological assessments and analyses. All authors reviewed and commented on the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The written informed consent was obtained before clinical sampling of the AM and adipose tissue. The use of the AM samples and laboratory animals was approved by the Ethics Committee of Mazandaran University of Medical Sciences (Ethic code: IR.MAZUMS.4.REC.1400.13758). Methods were performed according to ARRIVE guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalatbary, A.R., Omraninava, M., Nasiry, D. et al. Exosomes derived from human adipose mesenchymal stem cells loaded bioengineered three-dimensional amniotic membrane-scaffold-accelerated diabetic wound healing. Arch Dermatol Res 315, 2853–2870 (2023). https://doi.org/10.1007/s00403-023-02709-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-023-02709-z