Abstract
Introduction
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis that affects approximately 0.3–6 out of every 100,000 people worldwide. Clinical trials are scarce but there is growing interest in using newer and more targeted therapeutics to achieve disease remission. However, there are no standardized instruments to measure outcomes in PG and, therefore, future clinical trials are hampered by the absence of established and accurate means of assessment and comparison. Therefore, we aim to produce an internationally accepted core outcome set (COS) that will overcome this obstacle. This protocol outlines our intended approach to achieve the first part of this process, establishing a core outcome domain set.
Methods
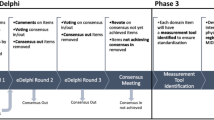
An international team of PG stakeholders, consisting of physicians, wound care nurses, patients, scientists and industry representatives, has been assembled for the purpose of building a comprehensive and universally established set of core outcome domains. During the first step, we will generate items of relevance using a nominal process from all stakeholders. Items will be distilled and collapsed into potential domains and subdomains. A systematic review of current methods for reporting PG has already been published and domains identified in this work will be considered in the generation of the core domains set. During the second step, after the potential domains and subdomains are identified, stakeholders will participate in an e-Delphi exercise to rate the importance of (sub)domains. A final consensus meeting will be organized with the goal of establishing a core domain set.
Conclusion
Pyoderma gangrenosum lacks an established COS and previously published clinical trials have used inconsistent measures established from similarly inconsistent domains. As a first step this study seeks to create a core domain set within the COS, to build the foundation for future core outcome work for PG.

Similar content being viewed by others
References
Maverakis E, Marzano AV, Le ST et al (2020) Pyoderma gangrenosum. Nat Rev Dis Prim 6(1):80. https://doi.org/10.1038/s41572-020-00221-6
Ormerod AD, Augustin M, Baker C et al (2012) Challenges for synthesising data in a network of registries for systemic psoriasis therapies. Dermatology 224(3):236–243. https://doi.org/10.1159/000338572
Thorlacius L, Ingram JR, Garg A et al (2017) Protocol for the development of a core domain set for hidradenitis suppurativa trial outcomes. BMJ Open. https://doi.org/10.1136/bmjopen-2016-014733
Schmitt J, Apfelbacher C, Spuls PI et al (2015) The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol 135(1):24–30. https://doi.org/10.1038/jid.2014.320
Kottner J, Jacobi L, Hahnel E et al (2018) Core outcome sets in dermatology: report from the second meeting of the International Cochrane Skin Group Core Outcome Set Initiative. Br J Dermatol 178(4):e279–e285. https://doi.org/10.1111/bjd.16324
Prinsen CAC, Spuls PI, Kottner J et al (2019) Navigating the landscape of core outcome set development in dermatology. J Am Acad Dermatol 81(1):297–305. https://doi.org/10.1016/j.jaad.2019.03.009
Eleftheriadou V, Thomas K, van Geel N et al (2015) Developing core outcome set for vitiligo clinical trials: international e-Delphi consensus. Pigment Cell Melanoma Res 28(3):363–369. https://doi.org/10.1111/pcmr.12354
Keeley T, Williamson P, Callery P et al (2016) The use of qualitative methods to inform Delphi surveys in core outcome set development. Trials. https://doi.org/10.1186/s13063-016-1356-7
Niederberger M, Spranger J (2020) Delphi technique in health sciences: a map. Front Public Heal 8:457. https://doi.org/10.3389/fpubh.2020.00457
Cochrane Skin – Core outcome set initiative. http://cs-cousin.org/. Accessed Jan 22, 2022.
Lopez-Olivo MA, Negrón JB, Zogala RJ et al (2019) Core outcome sets specifically for longterm observational studies: OMERACT special interest group update in rheumatoid arthritis. J Rheumatol 46(9):1164–1167. https://doi.org/10.3899/JRHEUM.181076
Kirkham JJ, Davis K, Altman DG et al (2017) Core outcome set-STAndards for development: the COS-STAD recommendations. PLOS Med 14(11):e1002447. https://doi.org/10.1371/JOURNAL.PMED.1002447
COMET Initiative | Home. https://www.comet-initiative.org/. Accessed Jan 22, 2022
Lu JD, Hobbs MM, Huang WW, Ortega-Loayza AG, Alavi A (2020) Identification and evaluation of outcome measurement instruments in pyoderma gangrenosum: a systematic review*. Br J Dermatol 183(5):821–828. https://doi.org/10.1111/bjd.19027
Williamson PR, Altman DG, Bagley H et al (2017) The COMET handbook: version 1.0. Trials 18(Suppl 3):1–50. https://doi.org/10.1186/s13063-017-1978-4
COMET Initiative | Taxonomy with examples. https://www.comet-initiative.org/Resources/Taxonomy. Accessed May 15, 2021.
Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR (2018) A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 96:84–92. https://doi.org/10.1016/j.jclinepi.2017.12.020
Lange T, Kottner J, Weberschock T et al (2021) Outcome assessment in dermatology clinical trials and cochrane reviews: call for a dermatology-specific outcome taxonomy. J Eur Acad Dermatol Venereol 35(2):523–535. https://doi.org/10.1111/JDV.16854
George C, Deroide F, Rustin M (2019) Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med J R Coll Phys Lond 19(3):224–228. https://doi.org/10.7861/clinmedicine.19-3-224
Goodarzi H, Sivamani RK, Garcia MS et al (2012) Effective strategies for the management of pyoderma gangrenosum. Adv Wound Care 1(5):194–199. https://doi.org/10.1089/wound.2011.0339
Maverakis E, Ma C, Shinkai K et al (2018) Diagnostic criteria of ulcerative Pyoderma Gangrenosum a delphi consensus of international experts. JAMA Dermatol 154(4):461–466. https://doi.org/10.1001/jamadermatol.2017.5980
Ortega-Loayza AG, Friedman MA, Reese AM et al (2021) Molecular and cellular characterization of pyoderma gangrenosum: implications for the use of gene expression. J Invest Dermatol. https://doi.org/10.1016/J.JID.2021.08.431
Acquitter M, Plantin P, Kupfer I, Auvinet H, Marhadour T (2015) Anakinra improves pyoderma gangrenosum in psoriatic arthritis: a case report. Ann Intern Med 163(1):70–71. https://doi.org/10.7326/L15-5107
Chalmers I, Glasziou P (2009) Avoidable waste in the production and reporting of research evidence. Lancet 374(9683):86–89. https://doi.org/10.1016/S0140-6736(09)60329-9
Author information
Authors and Affiliations
Contributions
All authors contributed to the drafting of this manuscript, recruitment of patients and development of this protocol
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rick, J., Gould, L.J., Marzano, A.V. et al. The “Understanding Pyoderma Gangrenosum, Review and Assessment of Disease Effects (UPGRADE)” Project: a protocol for the development of the core outcome domain set for trials in pyoderma gangrenosum. Arch Dermatol Res 315, 983–988 (2023). https://doi.org/10.1007/s00403-022-02424-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-022-02424-1