Abstract
Background
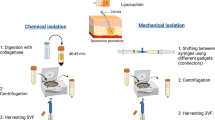
Stromal vascular fraction (SVF), derived enzymatically or mechanically from adipose tissue, contains a heterogenous population of cells and stroma, including multipotent stem cells. The regenerative capacity of SVF may potentially be adapted for a broad range of clinical applications, including the healing of acute cutaneous wounds.
Objective
To evaluate the available literature on the efficacy and safety of autologous adipose-derived stromal vascular fraction (SVF) for the treatment of acute cutaneous wounds in humans.
Methods
A systematic review of the literature utilizing MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials was performed to identify published clinical trials of autologous adipose-derived SVF or similar ADSC-containing derivatives for patients with acute cutaneous wounds. This was supplemented by searches for ongoing clinical trials through ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform.
Results
872 records were initially retrieved. Application of inclusion and exclusion criteria yielded 10 relevant studies: two completed non-randomized controlled trials and eight ongoing clinical trials. Both completed studies reported a statistically significant benefit in percentage re-epithelialization and time to healing for the SVF treatment arms. Safety information for SVF was not provided. Ongoing clinical trials were assessing outcomes such as safety, patient and observer reported scar appearance, wound healing rate, and wound epithelization.
Conclusion
In the context of substantial limitations in the quantity and quality of available evidence, the existing literature suggests that SVF may be a useful treatment for acute cutaneous wounds in humans. More clinical trials with improved outcome measures and safety assessment are needed.


Similar content being viewed by others
Availability of data and material
All data is presented in the manuscript.
References
Bourin P, Bunnell BA, Casteilla L et al (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International So. Cytotherapy 15(6):641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Nguyen A, Guo J, Banyard DA et al (2016) Stromal vascular fraction: a regenerative reality? Part 1: Current concepts and review of the literature. J Plast Reconstr Aesthetic Surg 69(2):170–179. https://doi.org/10.1016/j.bjps.2015.10.015
Aronowitz JA, Lockhart RA, Hakakian CS (2015) Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 4(1):1–9. https://doi.org/10.1186/s40064-015-1509-2
Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P (2010) Wound healing in the 21st century. J Am Acad Dermatol 63(5):866–881. https://doi.org/10.1016/j.jaad.2009.10.048
Zuk PA, Zhu M, Mizuno H et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228. https://doi.org/10.1089/107632701300062859
van Dongen JA, Stevens HP, Parvizi M, van der Lei B, Harmsen MC (2016) The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound repair Regen Off Publ Wound Heal Soc [and] Eur Tissue Repair Soc 24(6):994–1003. https://doi.org/10.1111/wrr.12482
van Dongen JA, Harmsen MC, Stevens HP (2019) Isolation of stromal vascular fraction by fractionation of adipose tissue. Methods Mol Biol 1993:91–103. https://doi.org/10.1007/978-1-4939-9473-1_8
van Dongen JA, Stevens HP, Harmsen MC, van der Lei B (2017) Mechanical micronization of lipoaspirates: squeeze and emulsification techniques. Plast Reconstr Surg 139(6):1369e–1370e. https://doi.org/10.1097/PRS.0000000000003372
Stevens HP, Donners S, de Bruijn J (2018) Introducing platelet-rich stroma: platelet-rich plasma (PRP) and stromal vascular fraction (SVF) combined for the treatment of androgenetic alopecia. Aesthetic Surg J 38(8):811–822. https://doi.org/10.1093/asj/sjy029
Janis JE, Harrison B (2014) Wound healing: Part I. Basic science. Plast Reconstr Surg 133(2):199e–207e. https://doi.org/10.1097/01.prs.0000437224.02985.f9
Martin P (1997) Wound healing--aiming for perfect skin regeneration. Science (80-). 276(5309):75LP–81. https://doi.org/10.1126/science.276.5309.75
Wu Y-L, Lin C-W, Cheng N-C, Yang K-C, Yu J (2017) Modulation of keratin in adhesion, proliferation, adipogenic, and osteogenic differentiation of porcine adipose-derived stem cells. J Biomed Mater Res B Appl Biomater 105(1):180–192. https://doi.org/10.1002/jbm.b.33551
Mizuno H, Tobita M, Uysal AC (2012) Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 30(5):804–810. https://doi.org/10.1002/stem.1076
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301. https://doi.org/10.1634/stemcells.2005-0342
Zonari A, Martins TMM, Paula ACC et al (2015) Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomater 17:170–181. https://doi.org/10.1016/j.actbio.2015.01.043
Valorani MG, Montelatici E, Germani A et al (2012) Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif 45(3):225–238. https://doi.org/10.1111/j.1365-2184.2012.00817.x
Ebrahimian TG, Pouzoulet F, Squiban C et al (2009) Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol 29(4):503–510. https://doi.org/10.1161/ATVBAHA.108.178962
Rehman J, Traktuev D, Li J et al (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109(10):1292–1298. https://doi.org/10.1161/01.CIR.0000121425.42966.F1
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83(3):835–870. https://doi.org/10.1152/physrev.2003.83.3.835
Zhao J, Hu L, Liu J, Gong N, Chen L (2013) The effects of cytokines in adipose stem cell-conditioned medium on the migration and proliferation of skin fibroblasts in vitro. Biomed Res Int 2013:578479. https://doi.org/10.1155/2013/578479
Cai L, Johnstone BH, Cook TG et al (2007) Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells 25(12):3234–3243. https://doi.org/10.1634/stemcells.2007-0388
Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH (2011) Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol 131(7):1559–1567. https://doi.org/10.1038/jid.2011.64
Wang L, Hu L, Zhou X et al (2017) Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep 7(1):13321. https://doi.org/10.1038/s41598-017-12919-x
Hu L, Wang J, Zhou X et al (2016) Exosomes derived from human adipose mesenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep 6(1):32993. https://doi.org/10.1038/srep32993
Sen CK (2019) Human wounds and its burden: an updated compendium of estimates. Adv Wound Care 8(2):39–48. https://doi.org/10.1089/wound.2019.0946
Tarallo M, Fino P, Ribuffo D et al (2018) Liposuction aspirate fluid adipose-derived stem cell injection and secondary healing in fingertip injury: a pilot study. Plast Reconstr Surg 142(1):136–147. https://doi.org/10.1097/PRS.0000000000004506
Cervelli V, Gentile P, De Angelis B, et al (2011) Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res 6(2):103–111. https://www.embase.com/search/results?subaction=viewrecord&id=L51212123&from=export
Cicione C, Di Taranto G, Barba M et al (2016) In vitro validation of a closed device enabling the purification of the fluid portion of liposuction aspirates. Plast Reconstr Surg 137(4):1157–1167. https://doi.org/10.1097/PRS.0000000000002014
Draaijers LJ, Tempelman FRH, Botman YAM et al (2004) The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 113(7):1960–1967. https://doi.org/10.1097/01.prs.0000122207.28773.56
van de Kar AL, Corion LUM, Smeulders MJC, Draaijers LJ, van der Horst CMAM, van Zuijlen PPM (2005) Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg 116(2):514–522. https://doi.org/10.1097/01.prs.0000172982.43599.d6
Ghiasloo M, Lobato RC, Díaz JM, Singh K, Verpaele A, Tonnard P (2020) Expanding clinical indications of mechanically isolated stromal vascular fraction: A systematic review. Aesthetic Surg J 40(9):NP546–NP560. https://doi.org/10.1093/asj/sjaa111
Aronowitz JA, Lockhart RA, Hakakian CS, Hicok KC (2015) Clinical safety of stromal vascular fraction separation at the point of care. Ann Plast Surg 75(6):666–671. https://doi.org/10.1097/SAP.0000000000000594
Granel B, Daumas A, Jouve E et al (2015) Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: An open-label phase I trial. Ann Rheum Dis 74(12):2175–2182. https://doi.org/10.1136/annrheumdis-2014-205681
Tzouvelekis A, Paspaliaris V, Koliakos G et al (2013) A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med 11(1):171. https://doi.org/10.1186/1479-5876-11-171
Bickers DR, Lim HW, Margolis D et al (2006) The burden of skin diseases: 2004. A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 55(3):490–500. https://doi.org/10.1016/j.jaad.2006.05.048
Spear M (2014) Principles of wound care—back to the basics. Plast Surg Nurs 34(3):150–152. https://doi.org/10.1097/PSN.0000000000000057
Chouhan D, Dey N, Bhardwaj N, Mandal BB (2019) Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 216:119267. https://doi.org/10.1016/j.biomaterials.2019.119267
Marino G, Moraci M, Armenia E et al (2013) Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res 185(1):36–44. https://doi.org/10.1016/j.jss.2013.05.024
Del BM, Pozzi MR, Rovati L, Mazzola I, Erba G, Bonomi S (2014) Autologous fat grafting for scleroderma-induced digital ulcersan effective technique in patients with systemic sclerosis. Handchirurgie Mikrochirurgie Plast Chir 46(4):242–247. https://doi.org/10.1055/s-0034-1376970
Marangi GF, Pallara T, Cagli B et al (2014) Treatment of early-stage pressure ulcers by using autologous adipose tissue grafts. Plast Surg Int 2014:817283. https://doi.org/10.1155/2014/817283
Del PN, Di Luca G, Sambataro D et al (2015) Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with systemic sclerosis. Cell Transplant 24(11):2297–2305. https://doi.org/10.3727/096368914X685636
Stasch T, Hoehne J, Huynh T, De Baerdemaeker R, Grandel S, Herold C (2015) Débridement and autologous lipotransfer for chronic ulceration of the diabetic foot and lower limb improves wound healing. Plast Reconstr Surg 136(6):1357–1366. https://doi.org/10.1097/PRS.0000000000001819
Carstens MH, Gómez A, Cortés R et al (2017) Non-reconstructable peripheral vascular disease of the lower extremity in ten patients treated with adipose-derived stromal vascular fraction cells. Stem Cell Res 18:14–21. https://doi.org/10.1016/j.scr.2016.12.001
Funding
Northwestern University, Department of Dermatology, unrestricted research funds. No external funding sources were utilized in the drafting of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We do not have any other relevant conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, M.H., Kang, B.Y., Wong, C.C. et al. A systematic review of autologous adipose-derived stromal vascular fraction (SVF) for the treatment of acute cutaneous wounds. Arch Dermatol Res 314, 417–425 (2022). https://doi.org/10.1007/s00403-021-02242-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-021-02242-x