Abstract
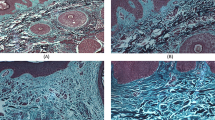
Dermal fibroblasts secrete various growth factors which are important for skin pigmentation. Imbalance in the synchronization of epidermal and dermal cells in the skin can play vital role in the pathogenesis of pigmentary disorder vitiligo. Therefore, our objective was to check the lesional fibroblasts for any abnormality and senescence in non-segmental vitiligo patients (NSV). Skin punch biopsies were taken from NSV patients and healthy controls. Explant culture of fibroblast from lesional dermis, non-lesional dermis, and control was analyzed. The senescence was confirmed by β-galactosidase staining in the cultured fibroblasts. Senescence was checked at mRNA level in lesional dermis, non-lesional dermis of NSV patients by senescence markers p16, p21, and hp1 by quantitative real-time polymerase chain reaction (qRT-PCR) and immunofluorescence study was used for protein analysis. Morphological results showed number of fibroblasts with bigger perinuclear region and vacuoles were more in the lesional fibroblasts. Number of β-galactosidase positive fibroblasts in the lesional skin of NSV patients was higher as compared to the non-lesional and control fibroblasts. Results showed higher relative gene expression of senescence markers p16, p21, and hp1 in the lesional dermis of NSV patients at mRNA level and protein level as compared with control. Senescence in the dermal fibroblasts can decrease the secretion of growth factors and cytokines secreted by fibroblasts which may lead to the melanocyte death and progression of vitiligo. However, further studies on larger number of patients are needed to confirm the role of fibroblasts in the vitiligo pathogenesis.




Similar content being viewed by others
References
Arias AM (2001) Epithelial mesenchymal interactions in cancer and development. Cell 105:425–431
Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI (1988) Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci USA 85:5112–5116
Bellei B, Pitisci A, Ottaviani M, Ludovici M, Cota C et al (2013) Vitiligo: a possible model of degenerative diseases. PLoS One 8(3):e59782
Camara-Lemarroy CR, Salas-Alanis JC (2013) The role of tumor necrosis factor-α in the pathogenesis of vitiligo. Am J Clin Dermatol 14:343
Cao L, Li W, Kim S, Brodie SG, Deng CX (2003) Senescence, aging and malignant transformation mediated by p53 in mice lacking the Brca1 full length isoform. Genes Dev 17(2):201–213
Castro P, Giri D, Lamb D, Ittmann M (2003) Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate 55(1):30–38
Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K et al (2004) Gene expression signature of fibroblast serum response predicts human cancer progression: Similarities between tumors and wounds. PLoS Biol 2:E7
Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C et al (2010) The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J Cell Sci 123:3102–3111
Clara CM, Graeme H, Joao F P (2014) Telomeres, oxidative stress and inflammatory factors: partners in cellular senescence? Longev Healthspan 3:1
Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M et al (2005) Tumour biology: senescence in premalignant tumours. Nat 436(7051):642.
Dell’Anna ML, Urbanelli S, Mastrofrancesco A, Camera E, Iacovelli P, Leone G et al (2003) Alterations of mitochondria in peripheral blood monoculear cells of vitiligo patients. Pigment Cell Res 16:553–559
Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA et al (2000) Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 20(4):1436–1447
Gauthier Y, Cario Andre M, Taieb A (2003) A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res 16:322–332
Giovannelli L, Bellandi S, Pitozzi V, Fabbri P, Dolara P, Moretti S (2004) Increased oxidative DNA damage in mononuclear leukocytes in vitiligo. Mutat Res 556:101–106
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C (1998). Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nat 391:357–362
Helenius M, Makelainen L, Salminen A (1999) Attenuation of NF-kappaB signaling response to UVB light during cellular senescence. Exp Cell Res 248:194–202
Imokawa G, Yada Y, Morisaki N, Kimura M (1998) Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. J Biochem 330:1235–1239
Itahana K, Campisi J, Dimri GP (2007) Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol 371:21–31
Kang MK, Bibb C, Baluda MA, Rey O, Park NH (2000) In vitro replication and differentiation of normal human oral keratinocytes. Exp Cell Res 258:288–297
Kitamura R, Tsukamoto K, Harada K, Shimizu A, Shimada S, Kobayashi T et al (2004) Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: role of SCF/KIT protein interactions and the downstream effector, MITF-M. J Pathol 202:463–475
Kosar M, Bartkova J, Hubackova S, Hodny Z, Lukas J, Bartek J (2011) Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type and insult-dependent manner, and follow expression of p16ink4a. Cell Cycle 10(3):457–468
Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L et al (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114:1299–1307
Lee AY, Kim NH, Choi WI, Youm YH (2005) Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. J Invest Dermatol 124:976–983
Lee AY, Youm YH, Kim NH, Yang H, Choi WI (2004) Keratinocytes in the depigmented epidermis of vitiligo are more vulnerable to trauma (suction) than keratinocytes in the normally pigmented epidermis, resulting in their apoptosis. Br J Dermatol 151:995–1003
Loughran O, Malliri A, Owens D, Gallimore PH, Stanley MA, Ozanne B et al (1996) Association of CDKN2A/p16INK4A with human head and neck keratinocyte replicative senescence: relationship of dysfunction to immortality and neoplasia. Oncogene 13(3):561–568
Matsumoto K, Okazaki H, Nakamura T (1992) Up-regulation of hepatocyte growth factor gene expression by interleukin-1 in human skin fibroblasts. Biochem Biophys Res Commun 188:235–243
Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF (2004) Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65:510–520
Meyskens FL Jr, Farmer P, Fruehauf JP (2001) Redox regulation in human melanocytes and melanoma. Pigment Cell Res 14(3):148–154
Mildner M, Mlitz V, Gruber F, Wojta J, Tschachler E (2007) Hepatocyte growth factor establishes autocrine and paracrine feedback loops for the protection of skin cells after UV irradiation. J Invest Dermatol 127:2637–2644
Moretti S, Fabbri P, Baroni G, Berti S, Bani D, Berti E et al (2009) Keratinocyte dysfunction in vitiligo epidermis: cytokine microenvironment and correlation to keratinocyte apoptosis. Histol Histopathol 24:849–857
Moretti S, Spallanzani A, Amato L, Hautmann G, Gallerani I, Fabiani M et al (2002) New insight into the pathogenesis of vitiligo: Imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res 15:87–92
Oh SH, Kim JY, Kim MR, Do JE, Shin JY, Hann SK (2012) DKK1 is highly expressed in the dermis of vitiligo lesion: is there association between DKK1 and vitiligo? J Dermatol Sci 66:163–165
Paratore C, Goerich DE, Suter U, Wegner M, Sommer L (2001) Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development 128:3949–3961
Purpura V, Persechino F, Belleudi F, Scrofani C, Raffa S, Persechino S et al (2014) Decreased expression of KGF/FGF7 and its receptor in pathological hypopigmentation. J Cell Mol Med 18:2553–2557
Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H et al (2002) A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol 22(19):5157–5172
Sato N, Maehara N, Goggins M (2004) Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res 64:6950–6956
Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC et al (1999) In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Invetsig Dermatol Symp Proc 4:91–96
Schulz I, Mahler HC, Boiteux S, Epe B (2000) Oxidative DNA base damage induced by singlet oxygen and photosensitization: Recognition by repair endonucleases and mutagenicity. Mutation res 461(2):145–156
Serrano M, Lee H, Chin L, Cordon-Cardo C, Beacg D, DePinho RA (1996) Role of the Ink4a locus in tumor suppression and cell mortality. Cell 85:27–37
Singh M, Mansuri MS, Parasrampuria MA, Begum R (2016) Interleukin 1-α: A modulator of melanocyte homeostasis in vitiligo. Biochem Anal Biochem 5:273
Sun LQ, Lee DW, Zhang Q, Xiao W, Raabe EH, Meeker A et al (2004) Growth retardation and premature aging phenotypes in mice with disruption of the SNF2-like gene. Genes Dev 18(9):1035–1046
Susuma O (2007) Abnormal mitosis and aberrant nuclear morphology in senescent human fibroblasts. In: Garvey RB (eds) New research on cell aging. Nova Science Publishers, New York, pp 65–81
Taïeb A, Picardo M (2010) Epidemiology, definitions and classification. In: Picardo Mauro (ed) Vitiligo, springer, Berlin, pp 13–24
Wonseon C, Rainer W, Wolfram G, Tobias M, Jan B, Christoph S et al (2010) The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J Cell Sci 123:3102–3111
Acknowledgements
We acknowledge DST-INSPIRE, New Delhi [IFA11-LSBM-61], [IF140212] for providing the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interests.
Funding
This study was supported by research grant from DST-INSPIRE Faculty Project, New Delhi [IFA11-LSBM-61] to Ravinder Kumar, and DST-INSPIRE fellowship [IF140212] to Seema Rani.
Ethical approval
Skin punch biopsy was collected from pigmentary Clinic of the Department of Dermatology of the Postgraduate Institute of Medical Education and Research, Chandigarh with informed consent of the patients after the approval by ethical committee of the institute.
Rights and permissions
About this article
Cite this article
Rani, S., Bhardwaj, S., Srivastava, N. et al. Senescence in the lesional fibroblasts of non-segmental vitiligo patients. Arch Dermatol Res 309, 123–132 (2017). https://doi.org/10.1007/s00403-016-1713-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-016-1713-0