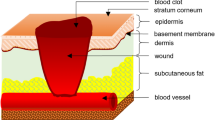
Abstract
Transforming growth factor-β (TGF-β) is a cytokine occurring in three isoforms with an important function in development and wound healing. In wound healing, prolonged TGF-β signaling results in myofibroblast differentiation and fibrosis. In contrast, the developing second-trimester fetal skin contains high levels of all three TGF-β isoforms but still has the intrinsic capacity to heal without scarring. Insight into TGF-β signal transduction during fetal wound healing might lead to methods to control the signaling pathway during adult wound healing. In this study, we imitated wound healing in vitro by stimulating fibroblasts with TGF-β1 and examining myofibroblast differentiation. The aim was to gain insight into TGF-β signaling in human fibroblasts from fetal and adult dermis. First, TGF-β1 stimulation resulted in similar or even more severe upregulation of myofibroblast-associated genes in fetal fibroblasts compared to adult fibroblasts. Second, fetal fibroblasts also had higher protein levels of myofibroblast-marker α-smooth muscle actin (α-SMA). Third, stimulated fetal fibroblasts in collagen matrices had higher protein levels of α-SMA, produced more of the fibrotic protein fibronectin splice-variant extra domain A (FnEDA), and showed enhanced contraction. Finally, fetal fibroblasts also produced significant higher levels of TGF-β1. Altogether, these data show that in vitro cultured fetal fibroblasts have myofibroblast-associated characteristics and do produce a fibrotic environment. As healthy fetal skin has high levels of TGF-β1, FnEDA, and collagen-III as well, these findings correlate with the in vivo situation. Therefore, our study demonstrates that there are similarities between fetal skin development and fibrosis and shows the necessity to discriminate between these processes.




Similar content being viewed by others
References
Arora PD, Narani N, McCulloch CA (1999) The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol 154:871–882
Balaji S, King A, Marsh E, LeSaint M, Bhattacharya SS, Han N, Dhamija Y, Ranjan R, Le LD, Bollyky PL, Crombleholme TM, Keswani SG (2015) The role of interleukin-10 and hyaluronan in murine fetal fibroblast function in vitro: implications for recapitulating fetal regenerative wound healing. PLoS One 10:e0124302
Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK (2007) Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Biol Cell 18:2716–2727
Biggs LC, Goudy SL, Dunnwald M (2015) Palatogenesis and cutaneous repair: a two-headed coin. Dev Dyn 244:289–310
Bruno G, Cencetti F, Pertici I, Japtok L, Bernacchioni C, Donati C, Bruni P (2015) CTGF/CCN2 exerts profibrotic action in myoblasts via the up-regulation of sphingosine kinase-1/S1P3 signaling axis: implications in the action mechanism of TGFbeta. Biochim Biophys Acta 1851:194–202
Carre AL, James AW, MacLeod L, Kong W, Kawai K, Longaker MT, Lorenz HP (2010) Interaction of wingless protein (Wnt), transforming growth factor-beta1, and hyaluronan production in fetal and postnatal fibroblasts. Plast Reconstr Surg 125:74–88
Cha J, Kwak T, Butmarc J, Kim TA, Yufit T, Carson P, Kim SJ, Falanga V (2008) Fibroblasts from non-healing human chronic wounds show decreased expression of beta ig-h3, a TGF-beta inducible protein. J Dermatol Sci 50:15–23
Colwell AS, Krummel TM, Longaker MT, Lorenz HP (2006) Fetal and adult fibroblasts have similar TGF-beta-mediated, Smad-dependent signaling pathways. Plast Reconstr Surg 117:2277–2283
Cukierman E, Pankov R, Yamada KM (2002) Cell interactions with three-dimensional matrices. Curr Opin Cell Biol 14:633–639
Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT (2005) Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen 13:198–204
Darby I, Skalli O, Gabbiani G (1990) Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63:21–29
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122:103–111
Desmouliere A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56–66
Fringer J, Grinnell F (2001) Fibroblast quiescence in floating or released collagen matrices: contribution of the ERK signaling pathway and actin cytoskeletal organization. J Biol Chem 276:31047–31052
Ghosh AK, Vaughan DE (2012) PAI-1 in tissue fibrosis. J Cell Physiol 227:493–507
Glim JE, Niessen FB, Everts V, van Egmond M, Beelen RH (2013) Platelet derived growth factor-CC secreted by M2 macrophages induces alpha-smooth muscle actin expression by dermal and gingival fibroblasts. Immunobiology 218:924–929
Gosiewska A, Yi CF, Brown LJ, Cullen B, Silcock D, Geesin JC (2001) Differential expression and regulation of extracellular matrix-associated genes in fetal and neonatal fibroblasts. Wound Repair Regen 9:213–222
Hanasono MM, Kita M, Mikulec AA, Lonergan D, Koch RJ (2003) Autocrine growth factor production by fetal, keloid, and normal dermal fibroblasts. Arch Facial Plast Surg 5:26–30
Heng NH, Zahlten J, Cordes V, Ong MM, Goh BT, N’Guessan PD, Pischon N (2015) Effects of enamel matrix derivative and transforming growth factor-beta1 on connective tissue growth factor in human periodontal ligament fibroblasts. J Periodontol 86:569–577
Hinz B (2006) Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol 85:175–181
Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C (2001) Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12:2730–2741
Hinz B, Gabbiani G (2010) Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep 2:78
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170:1807–1816
Kishi K, Nakajima H, Tajima S (1999) Differential responses of collagen and glycosaminoglycan syntheses and cell proliferation to exogenous transforming growth factor beta 1 in the developing mouse skin fibroblasts in culture. Br J Plast Surg 52:579–582
Klass BR, Grobbelaar AO, Rolfe KJ (2009) Transforming growth factor beta1 signalling, wound healing and repair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. Postgrad Med J 85:9–14
Lee NJ, Wang SJ, Durairaj KK, Srivatsan ES, Wang MB (2000) Increased expression of transforming growth factor-beta1, acidic fibroblast growth factor, and basic fibroblast growth factor in fetal versus adult fibroblast cell lines. Laryngoscope 110:616–619
Li-Korotky HS, Hebda PA, Lo CY, Dohar JE (2007) Age-dependent differential expression of fibronectin variants in skin and airway mucosal wounds. Arch Otolaryngol Head Neck Surg 133:919–924
Moulin V, Tam BY, Castilloux G, Auger FA, O’Connor-McCourt MD, Philip A, Germain L (2001) Fetal and adult human skin fibroblasts display intrinsic differences in contractile capacity. J Cell Physiol 188:211–222
Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE (2003) Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol 162:149–160
Pratsinis H, Giannouli CC, Zervolea I, Psarras S, Stathakos D, Kletsas D (2004) Differential proliferative response of fetal and adult human skin fibroblasts to transforming growth factor-beta. Wound Repair Regen 12:374–383
Rolfe KJ, Richardson J, Vigor C, Irvine LM, Grobbelaar AO, Linge C (2007) A role for TGF-beta1-induced cellular responses during wound healing of the non-scarring early human fetus? J Invest Dermatol 127:2656–2667
Rollins BJ, O’Connell TM, Bennett G, Burton LE, Stiles CD, Rheinwald JG (1989) Environment-dependent growth inhibition of human epidermal keratinocytes by recombinant human transforming growth factor-beta. J Cell Physiol 139:455–462
Santibanez JF, Quintanilla M, Bernabeu C (2011) TGF-beta/TGF-beta receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 121:233–251
Schor SL, Ellis IR, Jones SJ, Woolston AM, Schor AM (2012) Bistable switch in migration stimulating factor expression: regulation by the concerted signalling of transforming growth factor-beta1 and the extracellular matrix. Int J Cancer 130:2024–2032
Shinde AV, Kelsh R, Peters JH, Sekiguchi K, Van De Water L, McKeown-Longo PJ (2015) The alpha4beta1 integrin and the EDA domain of fibronectin regulate a profibrotic phenotype in dermal fibroblasts. Matrix Biol 41:26–35
Thapa N, Lee BH, Kim IS (2007) TGFBIp/betaig-h3 protein: a versatile matrix molecule induced by TGF-beta. Int J Biochem Cell Biol 39:2183–2194
van den Bogaerdt AJ, van Zuijlen PP, van Galen M, Lamme EN, Middelkoop E (2002) The suitability of cells from different tissues for use in tissue-engineered skin substitutes. Arch Dermatol Res 294:135–142
van der Slot AJ, van Dura EA, de Wit EC, De Groot J, Huizinga TW, Bank RA, Zuurmond AM (2005) Elevated formation of pyridinoline cross-links by profibrotic cytokines is associated with enhanced lysyl hydroxylase 2b levels. Biochim Biophys Acta 1741:95–102
Verrecchia F, Chu ML, Mauviel A (2001) Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem 276:17058–17062
Visscher M, Narendran V (2014) The Ontogeny of Skin. Adv Wound Care (New Rochelle) 3:291–303
Wadman M (2005) Scar prevention: the healing touch. Nature 436:1079–1080
Walraven M, Beelen RH, Ulrich MM (2015) Transforming growth factor-beta (TGF-beta) signaling in healthy human fetal skin: a descriptive study. J Dermatol Sci 78:117–124
Walraven M, Gouverneur M, Middelkoop E, Beelen RH, Ulrich MM (2014) Altered TGF-beta signaling in fetal fibroblasts: what is known about the underlying mechanisms? Wound Repair Regen 22:3–13
Walraven M, Talhout W, Beelen RH, van Egmond M, Ulrich MM (2016) Healthy human second-trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair Regen 24:533–541
Walraven M, van Vliet SJ, Beelen RH, van Egmond M, Ulrich MM (2016) Blocking alpha1-integrin reverts the adhesive phenotype of adult fibroblasts towards a foetal-like migratory phenotype. Exp Dermatol 25:480–482
Wipff PJ, Rifkin DB, Meister JJ, Hinz B (2007) Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179:1311–1323
Acknowledgements
The authors thank J. Braam, BSc., for the work she did during her internship. Furthermore, we are grateful to the personnel of the Centre for Contraception, Sexual Health and Abortion (CASA) in Leiden and of the Rode Kruis Ziekenhuis and the Association of Dutch Burn Centers (ADBC), both in Beverwijk, for collecting and processing tissues. We thank Dr. Ingo Herschel and Dr. Leon Olde Damink from Matricel GmbH for kindly providing Novomaix. This work was supported by the Netherlands Institute of Regenerative Medicine (NIRM, FES0908).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was given for all donated tissues and collection was done according to national guidelines formulated by the federation of Dutch medical scientific societies (FMWV/FDMSS).
Rights and permissions
About this article
Cite this article
Walraven, M., Akershoek, J.J., Beelen, R.H.J. et al. In vitro cultured fetal fibroblasts have myofibroblast-associated characteristics and produce a fibrotic-like environment upon stimulation with TGF-β1: Is there a thin line between fetal scarless healing and fibrosis?. Arch Dermatol Res 309, 111–121 (2017). https://doi.org/10.1007/s00403-016-1710-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-016-1710-3