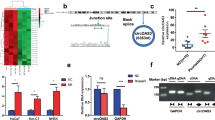
Abstract
Psoriasis is a chronic inflammatory skin disease that affects approximately 2–4% of the population. We recently described a novel non-coding RNA, psoriasis susceptibility related RNA gene induced by stress (PRINS), that was overexpressed in non-lesional psoriatic epidermis, and its expression was induced by various stress factors such as serum starvation, contact inhibition, ultraviolet (UV)-B irradiation, viral infection and translational inhibition in HaCaT cells. In the present work we set out to compare the stress and microbial agent-induced PRINS expression in normal human keratinocytes (NHKs) and HaCaT cells. Since nuclear factor-κB (NF-κB) is involved in the cellular stress response, we sought to explore whether there is a connection between the NF-κB and PRINS-mediated signal transduction pathways in NHKs and HaCaT cells. We found that the PRINS expression responded differentially to various stress signals and microbial agents in HaCaT cells and in NHKs: after translational inhibition and UV-B treatment, similar induction of PRINS expression occurred with different time courses while after microbial agent treatment, the PRINS expression was significantly induced in HaCaT cells, whereas we could not detect similar changes in NHKs. To explore whether the known NF-κB abnormalities in HaCaT cells could be related to this differential PRINS expression, we silenced the PRINS gene expression with small interfering RNA (siRNA) in both HaCaT cells and in NHKs and monitored NF-κB signal transduction after lipopolysaccharide (LPS) treatment. Silencing of PRINS had no effect on LPS-induced NF-κB activity either in HaCaT cells or in NHKs. Our results indicate that PRINS probably affects keratinocytes functions independently of NF-κB signalling.



Similar content being viewed by others
References
Bell S, Degitz K, Quirling M, Jilg N, Page S, Brand K (2003) Involvement of nf-kappab signalling in skin physiology and disease. Cell Signal 15(1):1–7
Bender K, Gottlicher M, Whiteside S, Rahmsdorf HJ, Herrlich P (1998) Sequential DNA damage-independent and -dependent activation of nf-kappab by uv. EMBO J 17(17):5170–5181
Bhalerao J, Bowcock AM (1998) The genetics of psoriasis: a complex disorder of the skin and immune system. Hum Mol Genet 7(10):1537–1545
Bos JD, Hulsebosch HJ, Krieg SR, Bakker PM, Cormane RH (1983) Immunocompetent cells in psoriasis in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res 275(3):181–189
Chaturvedi V, Qin JZ, Denning MF, Choubey D, Diaz MO, Nickoloff BJ (2001) Abnormal nf-kappab signaling pathway with enhanced susceptibility to apoptosis in immortalized keratinocytes. J Dermatol Sci 26(1):67–78
Cheriyath V, Leaman DW, Borden EC (2010) Emerging roles of fam14 family members (g1p3/isg 6–16 and isg12/ifi27) in innate immunity and cancer. J Interferon Cytokine Res 31(1):173–181
Collins LJ, Penny D (2009) The rna infrastructure: dark matter of the eukaryotic cell? Trends Genet 25(3):120–128
Duffin KC, Chandran V, Gladman DD, Krueger GG, Elder JT, Rahman P (2008) Genetics of psoriasis and psoriatic arthritis: update and future direction. J Rheumatol 35(7):1449–1453
Ghosh S, Karin M (2002) Missing pieces in the nf-kappab puzzle. Cell 109(Suppl):S81–S96
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S (1999) Cutting edge: toll-like receptor 4 (tlr4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for tlr4 as the lps gene product. J Immunol 162(7):3749–3752
Kis K, Bodai L, Polyanka H, Eder K, Pivarcsi A, Duda E, Soos G, Bata-Csorgo Z, Kemeny L (2006) Budesonide, but not tacrolimus, affects the immune functions of normal human keratinocytes. Int Immunopharmacol 6(3):358–368
Lewis DA, Hengeltraub SF, Gao FC, Leivant MA, Spandau DF (2006) Aberrant nf-kappab activity in hacat cells alters their response to uv-b signaling. J Invest Dermatol 126(8):1885–1892
Li N, Karin M (1998) Ionizing radiation and short wavelength uv activate nf-kappab through two distinct mechanisms. Proc Natl Acad Sci 95(22):13012–13017
Maas-Szabowski N, Starker A, Fusenig NE (2003) Epidermal tissue regeneration and stromal interaction in hacat cells is initiated by tgf-alpha. J Cell Sci 116(Pt 14):2937–2948
Mercurio F, Young DB, Manning AM (2000) Detection and purification of a multiprotein kinase complex from mammalian cells. Ikk signalsome. Methods Mol Biol 99:109–125
Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok PY, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR (2009) Genome-wide scan reveals association of psoriasis with il-23 and nf-kappab pathways. Nat Genet 41(2):199–204
Olaru F, Jensen LE (2010) Chemokine expression by human keratinocyte cell lines after activation of toll-like receptors. Exp Dermatol 19(8):e314–e316
Pivarcsi A, Bodai L, Rethi B, Kenderessy-Szabo A, Koreck A, Szell M, Beer Z, Bata-Csorgoo Z, Magocsi M, Rajnavolgyi E, Dobozy A, Kemeny L (2003) Expression and function of toll-like receptors 2 and 4 in human keratinocytes. Int Immunol 15(6):721–730
Pivarcsi A, Szell M, Kemeny L, Dobozy A, Bata-Csorgo Z (2001) Serum factors regulate the expression of the proliferation-related genes alpha5 integrin and keratin 1, but not keratin 10, in hacat keratinocytes. Arch Dermatol Res 293(4):206–213
Sagoo GS, Cork MJ, Patel R, Tazi-Ahnini R (2004) Genome-wide studies of psoriasis susceptibility loci: a review. J Dermatol Sci 35(3):171–179
Schneider RJ, Mohr I (2003) Translation initiation and viral tricks. Trends Biochem Sci 28(3):130–136
Seitz CS, Lin Q, Deng H, Khavari PA (1998) Alterations in nf-kappab function in transgenic epithelial tissue demonstrate a growth inhibitory role for nf-kappab. Proc Natl Acad Sci 95(5):2307–2312
Sonkoly E, Bata-Csorgo Z, Pivarcsi A, Polyanka H, Kenderessy-Szabo A, Molnar G, Szentpali K, Bari L, Megyeri K, Mandi Y, Dobozy A, Kemeny L, Szell M (2005) Identification and characterization of a novel, psoriasis susceptibility-related non-coding RNA gene, PRINS. J Biol Chem 280(25):24159–24167
Sur I, Ulvmar M, Toftgard R (2008) The two-faced nf-kappab in the skin. Int Rev Immunol 27(4):205–223
Szegedi K, Sonkoly E, Nagy N, Nemeth IB, Bata-Csorgo Z, Kemeny L, Dobozy A, Szell M (2010) The anti-apoptotic protein g1p3 is overexpressed in psoriasis and regulated by the non-coding RNA, PRINS. Exp Dermatol 19(3):269–278
Szell M, Bata-Csorgo Z, Kemeny L (2008) The enigmatic world of mrna-like ncrnas: Their role in human evolution and in human diseases. Semin Cancer Biol 18(2):141–148
Terui T, Ozawa M, Tagami H (2000) Role of neutrophils in induction of acute inflammation in t-cell-mediated immune dermatosis, psoriasis: A neutrophil-associated inflammation-boosting loop. Exp Dermatol 9(1):1–10
Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A (1999) The toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401(6755):811–815
Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM (1996) Suppression of tnf-alpha-induced apoptosis by nf-kappab. Science 274(5288):787–789
Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D (1999) Cutting edge: Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J Immunol 163(1):1–5
Acknowledgments
This work was supported by OTKA (NK77434), TÁMOP-4.2.1/B-09/1/KONV-2010-0005 and TÁMOP 4.2.2-08/1-2008.0001. We thank N. E. Fusenig (German Cancer Research Center, Heidelberg, Germany) and Zsuzsanna Győrfy (Institute of Biochemistry, Biological Research Center, Hungarian Academy of Sciences, Szeged, Hungary) for providing cell lines and luciferase reporter gene construct used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lilla Bari and Sarolta Bacsa contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bari, L., Bacsa, S., Sonkoly, E. et al. Comparison of stress-induced PRINS gene expression in normal human keratinocytes and HaCaT cells. Arch Dermatol Res 303, 745–752 (2011). https://doi.org/10.1007/s00403-011-1162-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-011-1162-8