Abstract
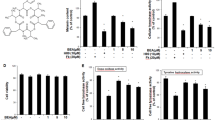
Melanogenesis is a physiological process that results in the synthesis of melanin pigments, which play a crucial protective role against skin photocarcinogenesis. The present study was designed to determine the effects of 6-benzylaminopurine (6-BAP) on melanogenesis and elucidate the molecular events of melanogenesis induced by 6-BAP. To elucidate the pigmenting effect of 6-BAP and its mechanism, several experiments were performed in B16 melanoma cells. Melanin content, tyrosinase activity, cAMP production, and Western blots for proteins which are involved in melanogenesis were introduced in this study. Melanin content and tyrosinase activity increased in response to treatment with 6-BAP in a concentration-dependent manner. The tyrosinase, TRP-1, TRP-2 and MITF protein levels were found to increase significantly in response to 6-BAP in a time-dependent manner. In addition, Western blot analysis revealed that 6-BAP increased the phosphorylated level of CRE-binding protein. The increased melanin synthesis that was induced by treatment with 6-BAP treatment was reduced significantly in response to co-treatment with H-89 [a protein kinase A (PKA) inhibitor], whereas co-treatment with SB203580 (a p38 MAPK inhibitor) and Ro-32-0432 (a PKC inhibitor) did not attenuate the increase in melanin content levels that was induced by 6-BAP. In a cAMP production assay, 6-BAP did not increase the intracellular cAMP level. These findings suggest that 6-BAP activates PKA via a cAMP-independent pathway and subsequently stimulates melanogenesis by up-regulating MITF and tyrosinase expression.





Similar content being viewed by others
References
Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, Ballotti R (1998) Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol 42:827–835. doi:10.1083/jcb.142.3.827
Busca R, Bertolotto C, Ortonne JP, Ballotti R (1996) Inhibition of the phosphatidylinositol 3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell differentiation. J Biol Chem 271:31824–31830. doi:10.1074/jbc.271.50.31824
Dulin NO, Niu J, Browning DD, Ye RD, Voyno-Yasenetskaya T (2001) Cyclic AMP-independent activation of protein kinase A by vasoactive peptides. J Biol Chem 276:20827–20830. doi:10.1074/jbc.C100195200
Englaro W, Bertolotto C, Busca R, Brunet A, Pages G, Ortonne JP, Ballotti R (1998) Inhibition of the mitogen-activated protein kinase pathway triggers B16 melanoma cell differentiation. J Biol Chem 273:9966–9970. doi:10.1074/jbc.273.16.9966
Englaro W, Rezzonico R, Durand-Clement M, Lallemand D, Ortonne JP, Ballotti R (1995) Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells. J Biol Chem 270:24315–24320. doi:10.1074/jbc.270.41.24315
Fesq H, Brockow K, Strom K, Mempel M, Ring J, Abeck D (2001) Dihydroxyacetone in a new formulation—a powerful therapeutic option in vitiligo. Dermatology 203:241–243. doi:10.1159/000051757
Friedmann PS, Gilchrest BA (1987) Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol 133:88–94. doi:10.1002/jcp.1041330111
Gaggioli C, Busca R, Abbe P, Ortonne JP, Ballotti R (2003) Microphthalmia-associated transcription factor (MITF) is required but is not sufficient to induce the expression of melanogenic genes. Pigment Cell Res 16:374–382. doi:10.1034/j.1600-0749.2003.00057.x
Gordon PR, Gilchrest BA (1989) Human melanogenesis is stimulated by diacylglycerol. J Invest Dermatol 93:700–702. doi:10.1111/1523-1747.ep12319900
Gruber JR, Ohno S, Niles RM (1992) Increased expression of protein kinase C alpha plays a key role in retinoic acid-induced melanoma differentiation. J Biol Chem 267:13356–13360
Hunt G, Todd C, Cresswell JE, Thody AJ (1994) Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci 107(Pt 1):205–211
Jimenez M, Kameyama K, Maloy WL, Tomita Y, Hearing VJ (1988) Mammalian tyrosinase: biosynthesis, processing, and modulation by melanocyte-stimulating hormone. Proc Natl Acad Sci USA 85:3830–3834. doi:10.1073/pnas.85.11.3830
Kimura T, Doi K (2004) Depigmentation and rejuvenation effects of kinetin on the aged skin of hairless descendants of Mexican hairless dogs. Rejuvenation Res 7:32–39. doi:10.1089/154916804323105062
Kinoshita M, Hori N, Aida K, Sugawara T, Ohnishi M (2007) Prevention of melanin formation by yeast cerebroside in B16 mouse melanoma cells. J Oleo Sci 56:645–648
Lee J, Jung E, Park J, Jung K, Park E, Kim J, Hong S, Park J, Park S, Lee S, Park D (2005) Glycyrrhizin induces melanogenesis by elevating a cAMP level in b16 melanoma cells. J Invest Dermatol 124:405–411. doi:10.1111/j.0022-202X.2004.23606.x
Levy S (1992) Dihydroxyacetone-containing sunless or self-tanning lotions. J Am Acad Dermatol 27:989–993. doi:10.1016/0190-9622(92)70300-5
Levy S (2000) Tanning preparations. Dermatol Clin 18:591–596. doi:10.1016/S0733-8635(05)70209-6
Ma Y, Pitson S, Hercus T, Murphy J, Lopez A, Woodcock J (2005) Sphingosine activates protein kinase A type II by a novel cAMP-independent mechanism. J Biol Chem 280:26011–26017. doi:10.1074/jbc.M409081200
Maeda K, Fukuda M (1996) Arbutin: mechanism of its depigmenting action in human melanocyte culture. J Pharmacol Exp Ther 276:765–769
Nicklas G, Sugumaran M (1995) Detection of dopachrome isomerase on gels and membranes. Anal Biochem 230:248–253. doi:10.1006/abio.1995.1470
Park HY, Russakovsky V, Ohno S, Gilchrest BA (1993) The beta isoform of protein kinase C stimulates human melanogenesis by activating tyrosinase in pigment cells. J Biol Chem 268:11742–11749
Price ER, Horstmann MA, Wells AG, Weilbaecher KN, Takemoto CM, Landis MW, Fisher DE (1998) Alpha-melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol Chem 273:33042–33047. doi:10.1074/jbc.273.49.33042
Saha B, Singh SK, Sarkar C, Bera R, Ratha J, Tobin DJ, Bhadra R (2006) Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signaling to CREB. Pigment Cell Res 19:595–605. doi:10.1111/j.1600-0749.2006.00348.x
Saito H, Yasumoto K, Takeda K, Takahashi K, Yamamoto H, Shibahara S (2003) Microphthalmia-associated transcription factor in the Wnt signaling pathway. Pigment Cell Res 16:261–265. doi:10.1034/j.1600-0749.2003.00039.x
Smalley K, Eisen T (2000) The involvement of p38 mitogen-activated protein kinase in the alpha-melanocyte stimulating hormone (alpha-MSH)-induced melanogenic and anti-proliferative effects in B16 murine melanoma cells. FEBS Lett 476:198–202. doi:10.1016/S0014-5793(00)01726-9
Widlund HR, Fisher DE (2003) Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene 22:3035–3041. doi:10.1038/sj.onc.1206443
Zhang L, Duan CJ, Binkley C, Li G, Uhler MD, Logsdon CD, Simeone DM (2005) A transforming growth factor beta-induced Smad3/Smad4 complex directly activates protein kinase A. Mol Cell Biol 24:2169–2180. doi:10.1128/MCB.24.5.2169-2180.2004
Acknowledgments
This research was partially supported by a grant from the Korean Ministry of Knowledge and Economy through the Program for the Regional Innovation System (B0009310) and the Ministry of Education, Science Technology (MEST) and Korean Industrial Technology Foundation (KOTEF) through the Human Resource Training Project for Regional Innovation.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Kim and J. Lee contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, S., Lee, J., Jung, E. et al. 6-Benzylaminopurine stimulates melanogenesis via cAMP-independent activation of protein kinase A. Arch Dermatol Res 301, 253–258 (2009). https://doi.org/10.1007/s00403-008-0924-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-008-0924-4