Abstract
Infliximab is a monoclonal antibody directed against TNF-α. It has been approved for use in rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriatic arthritis and plaque-type psoriasis. In case reports, positive effects on pustular variants of psoriasis have also been reported. However, paradoxically, manifestation of pustular psoriasis and plaque-type psoriasis has been reported in patients treated with TNF antagonists including infliximab for other indications. Here, we report on 5 patients with chronic plaque-type psoriasis who developed palmoplantar pustulosis during or after discontinuation of infliximab therapy. In two of the five cases, manifestation of palmoplantar pustulosis was not accompanied by worsening of plaque-type psoriasis. Possibly, site-specific factors or a differential contribution of immunological processes modulated by TNF inhibitors to palmoplantar pustulosis and plaque-type psoriasis may have played a role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased expression of TNF-α has been identified as an important pathophysiological mechanism in different types of chronic inflammation including psoriasis and psoriatic arthritis. The knowledge about the central role of TNF-α in certain diseases has successfully been converted to the therapeutic level; over the last years TNF antagonizing agents such as the anti-TNF directed antibodies infliximab and adalimumab and the fusion protein etanercept have substantially improved the treatment of some of the most common chronic inflammatory conditions such as rheumatoid arthritis (RA). With 80% of the patients reaching at least a 75% reduction of their baseline skin symptoms after 10 weeks of therapy [14], infliximab is regarded as one of the most potent agents available at present for the treatment of psoriasis vulgaris. Other variants of psoriasis, such as pustular psoriasis have not been formally tested in larger clinical trials. However, in individual reports, positive effects of infliximab on generalized pustular psoriasis (GPP) with dramatic improvements in some cases have been reported [3, 13, 15]. Because this type of pustular psoriasis is believed to correspond to an extreme activation of psoriatic disease mechanisms, the high bioavailability of infliximab and its rapid onset of action following intravenous infusion have been used to explain the surprisingly fast decrease of pustule formation in patients treated with the agent. Paradoxically, on the other hand, manifestation of pustular psoriasis has been reported in patients treated with TNF antagonists including infliximab for other indications.
Palmoplantar pustulosis (PPP) has long been regarded as a localized variant of pustular psoriasis, although more recent epidemiological and genetic findings argue against this concept [2, 12]. Here, we report on five patients with chronic plaque-type psoriasis who developed PPP during or after discontinuation of infliximab therapy.
Cases and discussion
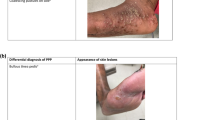
Relevant aspects of the five patients with chronic plaque-type psoriasis who developed PPP during or after discontinuation of infliximab therapy are presented in the Table 1 and Fig. 1.
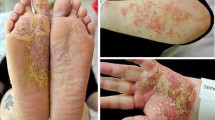
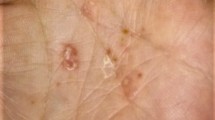
Clinical picture of pustulosis palmoplantaris in patient 3 with pustules in different stages of evolution on a sharply delineated erythematous lesion on the left sole (a) and yellowish pustules on the left palm (b). Histological examination showing intraepidermal vesiculopustular dermatitis (c, H.E. stain of a biopsy from the left plantar arch) with intraepidermal accumulation of neutrophils and subcorneal pustule formation (d)
To the best of our knowledge, the development of PPP during the treatment of plaque-type psoriasis with infliximab has not yet been reported. The occurrence of pustular skin lesions usually resembling GPP or palmoplantar pustular psoriasis has occasionally been observed in patients treated with infliximab for other indications [1, 6, 11, 16–19]. Induction of pustular skin lesions seems not to be limited to infliximab therapy, but has also been described in association with the use of the TNF-antagonists etanercept and adalimumab, including the use in one patient with plaque-psoriasis treated with etanercept [4, 8–10, 16]. One patient with seropositive RA developed GPP as well as PPP during treatment with infliximab [11]. This patient later experienced a relapse of PPP when treatment with etanercept was initiated, which also suggests that a class effect of TNF-antagonists may play a role.
In two of the three cases in whom an exacerbation of plaque-psoriasis occurred parallel to the manifestation of PPP, typical trigger factors for active psoriasis could be identified such as an infection (case 3) and the abrupt termination of anti-psoriatic treatment (case 2). These two cases are compatible with the existence of common trigger factors for plaque psoriasis and PPP. What are other factors that might contribute to the development of PPP during treatment of psoriasis vulgaris? While the exact etiology of PPP remains to be established, a history of smoking is the most important known risk factor for PPP. However, only one out of the three patients in whom a smoking history had been obtained was a smoker at the time of onset of pustular psoriasis (case 3). Streptococcal infection, a known risk factor for psoriasis vulgaris, has not been established as a risk factor for PPP and probably plays a minor role there. However, in the cases presented here, one patient (case 3) suffered an upper respiratory tract infection a few days before manifestation of PPP, while another patient (case 1) had suffered from a persistent cold 6 weeks before manifestation of pustules. In the former patient, the close temporal relationship between infectious symptoms and manifestation of PPP may point to a possible contribution of the infection to triggering PPP, and a modulation of the immune response to infliximab appears possible.
It is likely that beyond the contribution of known risk factors, other, immunological mechanisms may be involved in the manifestation of PPP under infliximab therapy. Interferon (IFN)-α has been suggested as a cytokine mediating the manifestation of psoriasiform lesions in patients treated with TNF-inhibitors as a consequence of crosstalk of TNF-α and IFN-α: TNF-α is known to suppress the generation of plasmacytoid dendritic cells that are very potent producers of IFN-α. Appearance of plasmacytoid dendritic cells (and IFN-α) in ths skin is considered to be an early and crucial step in the pathogenesis of psoriasis (reviewed in [7]). Thus, in patients treated with TNF-antagonists, the inhibition of TNF-α might induce an increase of IFN-α in the skin favoring the manifestation of psoriasiform dermatitis. In fact, an increase of IFN-α signaling has been shown in biopsy specimens from psoriatic plaques induced by TNF-inhibitors compared with traditional psoriatic plaques [6]. The relevance of IFN-α for PPP and TNF-inhibitor induced PPP, however, still needs to be determined. The observation of an improvement of pre-existing psoriasis plaques parallel to the first manifestation of PPP in two of the cases described here supports the concept that immunological mechanisms and/or local factors are not identical in the pathogenesis of plaque-type psoriasis and PPP. Differences in pathogenesis between plaque psoriasis and PPP are also supported by their different genetic background, with plaque psoriasis, but not PPP being linked to PSORS1, the major susceptibility locus for plaque-type psoriasis located on 6p21 [2]. The localized nature of the pustules on palms and soles occurring in association with infliximab therapy in some patients suggests a contribution of site-specific factors. Possibly, eccrine sweat glands that are numerous in the palmoplantar location are involved in these processes. Changes secondary to binding of infliximab to TNF-α, that has been shown to be expressed in eccrine sweat glands, have been suggested as a possible mechanism [11].
Withdrawal or dose reduction of systemic cyclosporine and systemic or topical glucocorticosteroids given for treatment of psoriasis vulgaris have been associated with manifestation of GPP, but not with PPP. To the best of our knowledge, no reports on manifestation of GPP or PPP associated with treatment with methotrexate, another drug frequently used in the therapy of psoriasis, exist. However, in the clinical trials with the CD11a-antagonist efalizumab in the indication plaque psoriasis, development of PPP has also been observed in 0.2% of 1,620 patients during the first 12 weeks of treatment with efalizumab, compared to none of the 715 patients in the placebo groups [5].
Whether therapy with TNF-antagonists may induce a more severe form of plaque psoriasis or psoriatic arthritis in some patients treated for these indications is difficult to determine, as the natural course of both plaque psoriasis and psoriatic arthritis is variable and a loss of effect is not easily differentiated from an induction of a more severe form of the respective disease. However, as a manifestation of plaque psoriasis has been noted in a number of patients treated with TNF-antagonists for other indications without a personal or family history of psoriasis [6, 16], it is likely that TNF-antagonists may in some individuals and probably in conjunction with environmental factors favor manifestation or worsening of psoriatic skin and/or bone disease.
The treatment of patients with plaque-psoriasis developing PPP should be decided on an individual basis. In case plaque-psoriasis remains controlled under infliximab therapy, addition of topical therapy may be sufficient for treatment of PPP in some instances (case 4). However, in some cases, additional topical therapy may not be sufficient and UV-therapy or another systemic therapy may be necessary in addition to or as replacement of infliximab therapy (case 3). When manifestation of PPP is accompanied by worsening of plaque-psoriasis, discontinuation of infliximab therapy is advisable and initiation of other systemic antipsoriatic agents, such as cyclosporine or a different TNF-antagonist, may be necessary (cases 1 and 5).
In summary, pustular psoriasis may show a good response to treatment with TNF-antagonists such as infliximab. However, pustular psoriasis may also manifest during treatment of rheumatological diseases, and, as described here, also in patients with plaque-type psoriasis under treatment with infliximab. Manifestation of PPP under infliximab is not necessarily accompanied by worsening of pre-existing plaque psoriasis. Management of the pustular skin lesions has to be decided on an individual basis. In a subgroup of patients, therapy with the TNF-antagonist has to be discontinued and another systemic therapy (including a different TNF-antagonist) will probably be necessary to sufficiently control plaque-psoriasis and PPP.
References
Adams DR, Buckel T, Sceppa JA (2006) Infliximab associated new-onset psoriasis. J Drugs Dermatol 5:178–179
Asumalahti K, Ameen M, Suomela S, Hagforsen E, Michaelsson G, Evans J, Munro M, Veal C, Allen M, Leman J, David BA, Kirby B, Connolly M, Griffiths CE, Trembath RC, Kere J, Saarialho-Kere U, Barker JN (2003) Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. J Invest Dermatol 120:627–632
Benoit S, Toksoy A, Brocker EB, Gillitzer R, Goebeler M (2004) Treatment of recalcitrant pustular psoriasis with infliximab: effective reduction of chemokine expression. Br J Dermatol 150:1009–1012
Beuthien W, Mellinghoff HU, von Kempis J (2004) Skin reaction to adalimumab. Arthritis Rheum 50:1690–1692
Carey W, Glazer S, Gottlieb AB, Lebwohl M, Leonardi C, Menter A, Papp K, Rundle AC, Toth D (2006) Relapse, rebound, and psoriasis adverse events: an advisory group report. J Am Acad Dermatol 54:S171–S181
de Gannes GC, Ghoreishi M, Pope J, Russell A, Bell D, Adams S, Shojania K, Martinka M, Dutz JP (2007) Psoriasis and pustular dermatitis triggered by TNF-{alpha} inhibitors in patients with rheumatologic conditions. Arch Dermatol 143:223–231
Fiorentino DF (2007) The Yin and Yang of TNF-{alpha} inhibition. Arch.Dermatol 143:233–236
Flendrie M, Vissers WH, Creemers MC, de Jong EM, van de Kerkhof PC, van Riel PL (2005) Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis Res Ther 7:R666–R676
Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, Gaspari AA, Ling M, Weinstein GD, Nayak A, Gordon KB, Zitnik R (2003) A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 139:1627–1632
Kary S, Worm M, Audring H, Huscher D, Renelt M, Sorensen H, Stander E, Maass U, Lee H, Sterry W, Burmester GR (2006) New onset or exacerbation of psoriatic skin lesions in patients with definite rheumatoid arthritis receiving tumour necrosis factor alpha antagonists. Ann Rheum Dis 65:405–407
Michaelsson G, Kajermo U, Michaelsson A, Hagforsen E (2005) Infliximab can precipitate as well as worsen palmoplantar pustulosis: possible linkage to the expression of tumour necrosis factor-alpha in the normal palmar eccrine sweat duct? Br J Dermatol 153:1243–1244
Mössner R, Kingo K, Kleensang A, Kruger U, Konig IR, Silm H, Westphal GA, Reich K (2005) Association of TNF -238 and -308 promoter polymorphisms with psoriasis vulgaris and psoriatic arthritis but not with pustulosis palmoplantaris. J Invest Dermatol 124:282–284
Newland MR, Weinstein A, Kerdel F (2002) Rapid response to infliximab in severe pustular psoriasis, von Zumbusch type. Int J Dermatol 41:449–452
Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CE (2005) Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 366:1367–1374
Schmick K, Grabbe J (2004) Recalcitrant, generalized pustular psoriasis: rapid and lasting therapeutic response to antitumour necrosis factor-alpha antibody (infliximab). Br J Dermatol 150:367
Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A (2005) Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum 52:2513–2518
Starmans-Kool MJ, Peeters HR, Houben HH (2005) Pustular skin lesions in patients treated with infliximab: report of two cases. Rheumatol Int 25:550–552
Thurber M, Feasel A, Stroehlein J, Hymes SR (2004) Pustular psoriasis induced by infliximab. J Drugs Dermatol 3:439–440
Wegscheider BJ, El Shabrawi L, Weger M, Ardjomand N, Hermann J, Aberer E, El Shabrawi Y (2007) Adverse skin reactions to infliximab in the treatment of intraocular inflammation. Eye 21:547–549
Mrowietz U, Elder JT, Barker J (2006) The importance of disease associations and concomitant therapy for the long-term management of psoriasis patients. Arch Dermatol Res 298:309–319
Nast A, Kopp I, Augustin M, Banditt KB, Boehncke WH, Follmann M, Friedrich M, Huber M, Kahl C, Klaus J, Koza J, Kreiselmaier I, Mohr J, Mrowietz U, Ockenfels HM, Orzechowski HD, Prinz J, Reich K, Rosenbach T, Rosumeck S, Schlaeger M, Schmid-Ott G, Sebastian M, Streit V, Weberschock T, Rzany B (2007) German evidence-based guidelines for the treatment of psoriasis vulgaris (short version). Arch Dermatol Res 299:111–138
Papp KA (2006) The long-term efficacy and safety of new biological therapies for psoriasis. Arch.Dermatol.Res 298(1):7–15
Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M (2006) Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res 298:321–328
Conflict of interest
R. M. has received travel grants from Abbot, Essex and Wyeth. K. R. has served as a consultant and paid speaker for Abbot, Biogen Idec, Centocor, Essex, Schering-Plough, Serono, and Wyeth. He has also received grant funding from Biogen Idec.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mössner, R., Thaci, D., Mohr, J. et al. Manifestation of palmoplantar pustulosis during or after infliximab therapy for plaque-type psoriasis: report on five cases. Arch Dermatol Res 300, 101–105 (2008). https://doi.org/10.1007/s00403-008-0831-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-008-0831-8