Abstract
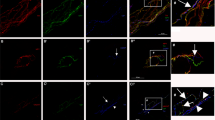
This study was undertaken to elucidate the morphological effects of histamine on subepidermal nerve fibers. A 10% histamine ointment was topically applied to the back skin of 17 adult male Hartley guinea pigs. Biopsy specimens were obtained at times from 5 min to 24 h, and were examined by conventional immunofluorescence (IF), laser scanning confocal fluorescence microscopy (LSCM) and transmission electron microscopy. On IF and LSCM, marked diminutions in the immunoreactivity of protein gene product 9.5-immunoreactive (PGP 9.5-IR) fibers as well as of substance P-immunoreactive (SP-IR) and calcitonin gene-related peptide-immunoreactive (CGRP-IR) substances were observed 5 min after histamine application. By 30 min, immunoreactivity of PGP 9.5, SP and CGRP was completely lost. By 2 h, however, immunoreactivity of PGP 9.5-IR fibers and CGRP-IR substances started to show recovery. By 4 h, immunoreactivity of PGP 9.5, SP and CGRP had almost recovered, but the recovery time for each substance was slightly different (PGP 9.5 first, CGRP next, and SP last). By 6 h after histamine application, immunoreactivity of all these substances had fully recovered. Ultrastructurally, 5 min after histamine application, axonal and mitochondrial swelling and glycogen deposition were seen in the axons of subepidermal nerve fibers. By 30 min, severe axonal degeneration had occurred in some of the axons. It was only by 4 to 6 h that almost normal ultrastructural features were observed. Schwann cells and perineurial cells did not show any pathological changes. These findings demonstrate that 10% histamine ointment produced organic changes in the axons in the subepidermal nerve fibers of guinea pig skin, but these morphological changes were short-lived, reversible and transitory.








Similar content being viewed by others
References
Babe KS, Serafin WE (1996) Histamine, bradykinin and their antagonists. In: Goodman and Gilman’s the pharmacological basis of therapeutics, 9th edn. McGraw-Hill, New York, pp 581–600
Bray GM, Peyronnard JM, Aguayo AJ (1972) Reactions of unmyelinated nerve fibers to injury: an ultrastructural study. Brain Res 42:297–309
Chan SY, Ochs S, Worth RM (1980) The requirement of calcium ions and the effect of other ions on axoplasmic transport in mammalian nerve. J Physiol 301:477–504
Constantinides P (1984) Ultrastructural pathobiology. Elsevier, Amsterdam New York, p 41
Dalsgaard C-J, Jonsson C-R, Hökfelt T, Cuello AC (1983) Localization of substance P-immunoreactive nerve fibers in the human digital skin. Experientia 39:1018–1020
Dalsgaard C-J, Rydh M, Haegerstrand A (1989) Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry 92:385–389
Doran JF, Jackson PK, Kynoch PAM, Thompson RJ (1983) Isolation of PGP 9.5, a new human neuron-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem 40:1542–1547
Ebertz JM, Hirshman CA, Kettelkamp NS, Uno H, Hanifin JM (1987) Substance P-induced histamine release in human cutaneous mast cells. J Invest Dermatol 88:682–685
Foreman JC, Jordan CC, Oehme P, Renner H (1983) Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol 335:449–465
Gamse R, Petsche U, Lembeck F, Jansco G (1982) Capsaicin applied to peripheral nerves inhibits axoplasmic transport of substance P and somatostatin. Brain Res 239:447–462
Ghadially FN (1997) Ultrastructural pathology of the cell and matrix, 4th edn, vol 2. Butterworth-Heinemann, Boston, pp 1022–1029
Gibbins IL, Wattchow D, Conventry B (1987) Two immunohistochemically identified populations of calcitonin gene-related peptide (CGRP)-immunoreactive axons in human skin. Brain Res 414:143–148
Greaves MW, Wall PD (1996) Pathophysiology of itching. Lancet 348:938–940
Hägemark O, Hökfelt T, Pernow B (1978) Flare and itch induced by substance P in human skin. J Invest Dermatol 71:233–235
Hordinsky MK, Kennedy W, Wendelschafer-Crabb G, Lewis S (1995) Structure and function of cutaneous nerves in alopecia areata. J Invest Dermatol 104 [5 Suppl]:28S–29S
Hordinsky MK, Ericson M, Snow D, Boeck C, Lee W-S (1999) Peribulbar innervation and substance P expression following nonpermanent injury to the human scalp hair follicle. J Invest Dermatol Symp Proc 4:316–319
Ishihara M, Endo R, Rivera MRP, Mihara M (2002) Bird’s eye view observations of the subepidermal nerve network of normal guinea pig skin. Arch Dermatol Res 294:281–285
Izumi S, Uemura S, Nagamura Y, Kamoshida S, Kawai K (2002) Practice of immunostaining in immunohistochemistry. In: Nakane H, Nagamura Y, Tsutsumi H (eds) Watanabe-Nakane Kouso Koutai Hou (Watanabe-Nakane immunohistochemistry), revised 4th edn (in Japanese). Gakusai kikaku, Tokyo, pp 191–193
Joó F, Szolcsányi J, Jancsó-Gàbor A (1969) Mitochondrial alterations in the spinal ganglion cells of the rat accompanying the long-lasting sensory disturbance induced by capsaicin. Life Sci 8:621–626
Karanth SS, Springall DR, Kuhn DM, Levene MM, Polak JM (1991) An immunocytochemical study of cutaneous innervation and the distribution of neuropeptides and protein gene product 9.5 in man and commonly employed laboratory animals. Am J Anat 191:369–383
Krogstad AL (1999) Neurogenic control of blood flow and histamine release in psoriatic skin. Acta Derm Venereol Suppl (Stockh) 203:1–43
Lange K (2000) Regulation of cell volume via microvillar ion channels. J Cell Physiol 185:21–35
Lemasters JJ, Nieminem AL, Qian T, Trost LC, Herman B (1997) The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol Cell Biochem 174:159–165
Lembeck F, Holzer P (1979) Substance P as a neurogenic mediator of antidromic vasodilatation and neurogenic plasma extravasation. Naunyn-Schmiedbergs Arch Pharmacol 310:175–183
Magerl W, Westerman RA, Möhner B, Handwerker HO (1990) Properties of transdermal histamine iontophoresis: differential effects of season, gender, and body region. J Invest Dermatol 94:347–352
Malmfors T, Sachs C (1968) Degeneration of adrenergic nerves produced by 6-hydroxydopamine. Eur J Pharmacol 3:89–92
Marsch SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D (1987 ) The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience 23:275–289
McLachlan EM, Keast JR, Bauer M (1994) SP- and CGRP-immunoreactive axons differ in their ability to reinnervate the skin of the rat tail. Neurosci Lett 176:147–151
Mihara M, Hashimoto K, Kumakiri M (1982) Intraepidermal free nerve endings relating to epidermal melanocytes in spotted guinea pigs: A statistical and electron microscopic study. J Dermatol 9:63–72
Mihara M, Hashimoto K, Kumakiri M (1982) Intraepidermal free nerve endings in guinea pigs. Their relationship to epidermal keratinocytes. J Dermatol 9:107–116
Papka RE, Furness JB, Della NG, Murphy R, Costa M (1984) Time course of effect of capsaicin on ultrastructure and histochemistry of substance P-immunoreactive nerves associated with the cardiovascular system of the guinea-pig. Neuroscience 12:1277–1292
Pini A, Baranowski R, Lynn B (1990) Long-term reduction in the number of C-fiber nociceptors following capsaicin treatment of a cutaneous nerve in adult rats. Eur J Neurosci 2:89–97
Piotrowski W, Foreman JC (1986) Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br J Dermatol 114:37–46
Reily MA, Schayer RW (1970) In vivo studies on histamine catabolism and its inhibition. Br J Pharmacol 38:478–489
Schlaepfer WW (1974) Calcium-induced degeneration of axoplasm in isolated segments of rat peripheral nerve. Brain Res 69:203–215
Ständer S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T (2003) Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol 139:1463–1470
Steinhoff M, Ständer S, Seeliger S, Ansel JC, Schmelz M (2003) Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol 139:1479–1488
Thomas PK, King RHM (1974) The degeneration of unmyelinated axons following nerve section: an ultrastructural study. J Neurocytol 3:497–512
Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J (1983) PGP 9.5—a new marker for vertebrate neurons and neuroendocrine cells. Brain Res 278:224–228
Acknowledgements
The authors would like to extend their sincere appreciation to: Dr. Ryota Teshima, Professor and Chairman, Division of Orthopaedic Surgery, Department of Medicine of Sensory and Motor Organs, Faculty of Medicine, Tottori University, for the use of the department’s laser scanning confocal microscope; Dr. Masahiko Ishihara, Associate Professor, Division of Dermatology, Department of Medicine of Sensory and Motor Organs, Faculty of Medicine, Tottori University, for suggestions on immunofluorescence; Dr. Fumie Tabuchi, Vice-director, Department of Hospital Pharmacy, Tottori University Hospital, for providing the histamine ointments; Mr. Taichi Shirao, Leica Microsystems, Osaka, Japan, for excellent technical assistance; and to all those who, in one way or another, helped in the completion of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivera, M.R.P., Mihara, M. Time course effect and sequential morphological changes of subepidermal nerve fibers in guinea pig skin after application of histamine ointment: a laser scanning confocal fluorescence microscopic and electron microscopic study. Arch Dermatol Res 295, 549–556 (2004). https://doi.org/10.1007/s00403-004-0459-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-004-0459-2