Abstract
Purpose
Surrogate outcomes are clinical endpoints that are used as substitutes for direct measures of how a patient feels, functions, or survives. The present study aims to analyze the impact of surrogate outcomes on the results of randomized controlled trials on shoulder rotator cuff tears disorders.
Methods
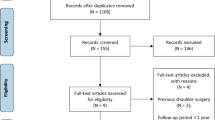
Randomized controlled trials (RCTs) related to rotator cuff tear conditions published up until 2021 were retrieved from the PubMed and ACCESSSS databases. The primary outcome of the article was considered a surrogate outcome when the authors used radiological, physiologic, or functional variables. The result of the article was considered positive when results supported the intervention based on the trial’s primary outcome. We recorded the sample size, the mean follow-up, and the type of funding. Statistical significance was set at p < 0.05.
Results
A total of 112 papers were included in the analysis. The mean sample size was 87.6 patients; mean follow-up period was 25.97 months. Thirty-six out of 112 RCTs used a surrogate outcome as a primary endpoint. More than half of papers using surrogate outcomes reported a positive finding (20 out of 36), while 10 out of 71 RCTs using patient-centered outcomes favored the intervention (14.08%, p < 0.001) [RR = 3.94 (95% CI 2.07–7.51)]. The mean sample size was smaller in trials using surrogate endpoints (75.11 vs 92.35 patients, respectively, p = 0.049), while the follow-up was shorter (14.12 m vs. 31.9 m, p < 0.001). Approximately 25% of the papers that reported surrogate endpoints (22.58%) were industry-funded projects.
Conclusions
The substitution of surrogate endpoints for patient-important outcomes in shoulder rotator cuff trials quadruplicates the chances of obtaining a favorable result that favors the analyzed intervention.

Similar content being viewed by others
References
Heneghan C, Goldacre B, Mahtani KR (2017) Why clinical trial outcomes fail to translate into benefits for patients. Trials 18:122. https://doi.org/10.1186/s13063-017-1870-2
Kim C, Prasad V (2015) Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration Approvals. JAMA Intern Med 175:1992–1994. https://doi.org/10.1001/jamainternmed.2015.5868
Rupp T, Zuckerman D (2017) Quality of life, overall survival, and costs of cancer drugs approved based on surrogate endpoints. JAMA Intern Med 177(2):276–277. https://doi.org/10.1001/jamainternmed.2016.7761. (PMID: 27898978)
Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. https://doi.org/10.1067/mcp.2001.113989
U.S. Food and Drug Administration Website. https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development. Accessed 20 Jan 2022
Callaham ML, Wears RL, Weber EJ, Barton C, Young G (1998) Positive-outcome bias and other limitations in the outcome of research abstracts submitted to a scientific meeting. JAMA 280:254–257
Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG (2004) Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 291:2457–2465. https://doi.org/10.1001/jama.291.20.2457
Dickersin K, Min YI, Meinert CL (1992) Factors influencing publication of research results. Follow-up of applications submitted to two institutional review boards. JAMA 267:374–378
Dwan K, Altman DG, Arnaiz JA et al (2008) Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS ONE 3:e3081. https://doi.org/10.1371/journal.pone.0003081
Hasenboehler EA, Choudhry IK, Newman JT, Smith WR, Ziran BH, Stahel PF (2007) Bias towards publishing positive results in orthopedic and general surgery: a patient safety issue? Patient Saf Surg 1:4. https://doi.org/10.1186/1754-9493-1-4
Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J (2013) Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS ONE 8:e75697. https://doi.org/10.1371/journal.pone.0075697
Treanor L, Frank RA, Cherpak LA, Dehmoobad Sharifabadi A, Salameh JP, Hallgrimson Z et al (2020) (2020) Publication bias in diagnostic imaging: conference abstracts with positive conclusions are more likely to be published. Eur Radiol 30:2964–2972. https://doi.org/10.1007/s00330-019-06568-z
Schneider P, Bransford R, Harvey E, Agel J (2019) Operative treatment of displaced midshaft clavicle fractures: has randomised control trial evidence changed practice patterns? BMJ Open 9:e031118. https://doi.org/10.1136/bmjopen-2019-031118
Hudgins JD, Fine AM, Bourgeois FT (2016) Effect of randomized clinical trial findings on emergency management. Acad Emerg Med 23:36–47. https://doi.org/10.1111/acem.12840
Franceschi F, Papalia R, Franceschetti E, Palumbo A, Del Buono A, Paciotti M et al (2016) Double-row repair lowers the retear risk after accelerated rehabilitation. Am J Sports Med 44:948–956. https://doi.org/10.1177/0363546515623031
Lapner P, Li A, Pollock JW, Zhang T, McIlquham K, McRae S et al (2021) Multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair: long-term follow-up. Am J Sports Med 49:3021–3029. https://doi.org/10.1177/03635465211029029
Johannsen AM, Arner JW, Elrick BP, Nolte PC, Rakowski DR, Horan MP et al (2021) Minimum 10-year outcomes of primary arthroscopic transosseous-equivalent double-row rotator cuff repair. Am J Sports Med 49:2035–2041. https://doi.org/10.1177/03635465211015419
Svensson S, Menkes DB, Lexchin J (2013) Surrogate outcomes in clinical trials: a cautionary tale. JAMA Intern Med 173(8):611–612. https://doi.org/10.1001/jamainternmed.2013.3037
Zumstein MA, Rumian A, Lesbats V, Schaer M, Boileau P (2014) Increased vascularization during early healing after biologic augmentation in repair of chronic rotator cuff tears using autologous leukocyte- and platelet-rich fibrin (L-PRF): a prospective randomized controlled pilot trial. J Shoulder Elbow Surg 23:3–12. https://doi.org/10.1016/j.jse.2013.08.017
Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB (2012) A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 28:8–15. https://doi.org/10.1016/j.arthro.2011.06.038
Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF (2012) The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med 40:1234–1241. https://doi.org/10.1177/0363546512442924
Bryant D, Holtby R, Willits K, Litchfield R, Drosdowech D, Spouge A et al (2016) A randomized clinical trial to compare the effectiveness of rotator cuff repair with or without augmentation using porcine small intestine submucosa for patients with moderate to large rotator cuff tears: a pilot study. J Shoulder Elbow Surg 25:1623–1633. https://doi.org/10.1016/j.jse.2016.06.006
Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM et al (1991) Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 325:1468–1475. https://doi.org/10.1056/NEJM199111213252103
Lamas JR, García-Fernández C, Tornero-Esteban P, Lópiz Y, Rodriguez-Rodriguez L, Ortega L et al (2019) Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full-thickness rotator cuff tears. Trials 20:387. https://doi.org/10.1186/s13063-019-3504-3
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval:
The study protocol was approved by the research committee (2022/0541).
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miquel, J., Salomó-Domènech, M., Santana, F. et al. Impact of surrogate outcomes in randomized controlled trials for shoulder rotator cuff tears. Arch Orthop Trauma Surg 143, 6117–6122 (2023). https://doi.org/10.1007/s00402-023-04911-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-04911-0