Abstract
Introduction
The different cartilage layers vary in synthesis of proteoglycan and of the distinct types of collagen with the predominant collagen Type II with its associated collagens, e.g. types IX and XI, produced by normal chondrocytes. It was demonstrated that proteoglycan decreases in degenerative tissue and a switch from collagen type II to type I occurs. The aim of this study was to evaluate the correlation of real-time (RT)-PCR and Photoshop-based image analysis in detecting such lesions and find new aspects about their distribution.
Patients
We performed immunohistochemistry and histology with cartilage tissue samples from 20 patients suffering from osteoarthritis compared with 20 healthy biopsies. Furthermore, we quantified our results on the gene expression of collagen type I and II and aggrecan with the help of real-time (RT)-PCR. Proteoglycan content was measured colorimetrically. Using Adobe Photoshop the digitized images of histology and immunohistochemistry stains of collagen type I and II were stored on an external data storage device. The area occupied by any specific colour range can be specified and compared in a relative manner directly from the histogram using the “magic wand tool” in the select similar menu. In the image grow menu gray levels or luminosity (colour) of all pixels within the selected area, including mean, median and standard deviation, etc. are depicted. Statistical Analysis was performed using the t test.
Method
With the help of immunohistochemistry, RT-PCR and quantitative RT- PCR we found that not only collagen type II, but also collagen type I is synthesized by the cells of the diseased cartilage tissue, shown by increasing amounts of collagen type I mRNA especially in the later stages of osteoarthritis.
Results
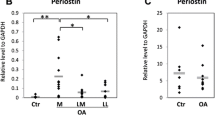
A decrease of collagen type II is visible especially in the upper fibrillated area of the advanced osteoarthritic samples, which leads to an overall decrease. Analysis of proteoglycan showed a loss of the overall content and a quite uniform staining in the different zones compared to the healthy cartilage with a classical zonal formation. Correlation analysis of the proteoglycan Photoshop measurements with the RT-PCR using Spearman correlation analysis revealed strong correlation for Safranin O and collagen type I, medium for collagen type II and glycoprotein but weak correlation between PCR aggrecan results.
Conclusion
Photoshop-based image analysis might become a valuable supplement for well known histopathological grading systems of lesioned articular cartilage.





Similar content being viewed by others
References
Kim TK, Sharma B, Wiliams CG, Ruffner MA, Malik A, Mc Farland EG, Elisseeff JH (2003) Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthr Cartil 11:653–664
Young AA, Smith MM, Smith SM, Cake MA, Ghosh P, Read RA et al (2005) Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res Ther 7(4):R852–R861
Gebhard PM, Gehrsitz A, Bau B, Soder S, Eger W, Aigner T (2003) Quantification of expression levels of cellular differentiation markers does not support a general shift in the cellular phenotype of osteoarthritic chondrocytes. J Orthop Res 21:96–101
Miosge N, Hartmann M, Maelicke C, Herken R (2004) Expression of collagen type I and type II in consecutive stages of human osteoarthritis. Histochem Cell Biol 122(3):229–236
Matkowsjyj KA, Cox R, Jensen RT, Benja RV (2003) Quantitative immunohistochemistry by measuring cumulative signal strength accurately measures receptor number. J Histochem Cytochem 51:205–214
Lehr HA, Mankoff DA, Corwin D, Santeusanio G, Gown AM (1997) Application of photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. J Histochem Cytochem 45:1559–1565
Lahm A, Uhl M, Lehr HA, Ihling C, Kreuz PC, Haberstroh J (2004) Photoshop based image analysis of canine articular cartilage after subchondral damage. Arch OrthopTrauma Surg 124:431–436
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RES0034
Baelde HJ, Cleton-Jansen AM, v Beerendonk H, Namba M, Bovee JVMG, Hogendoorn PCW (2001) High Quality RNA isolation from tumours with low cellularity and high extracellular matrix component for cDNA microarrays: application to chondrosarcoma. J Clin Path 54:778–782
Squires GR, Okouneff S, Ionescu M, Poole RA (2003) The pathobiology of focal lesions development in aging human articular cartilage and molecular matrix changes characteristics of osteoarthritis. Arthritis Rheum 48:1261–1270
Lorenz H, Wenz W, Ivancic M, Steck E, Richter W (2005) Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res Ther 7(1):R156–R165
Lorenz H, Richter W (2006) Osteoarthritis: cellular and molecular changes in degenerating cartilage. Prog Histochem Cytochem 40:135–163
Martin I, Jakob M, Schäfer D, Dick W, Spagnoli G, Heberer M (2001) Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthr Cartil 9:112–118
Lahm A, Kreuz PC, El Tayeh A, Mrosek E, Oberst M, Haberstroh J, Merk H (2007) Loss of zonal formation of articular cartilage in experimental early osteoarthritis. Saudi Med J 28 4:649–652
Bluteau G, Conrozier T, Mathieu P, Vignon E, Herbage D, Mallein-Gerin F (2001) Matrix metalloproteinase-1, -3, -13 and aggrecanase-1 and -2 are differentially expressed in experimental osteoarthritis. Biochim Biophys Acta 1526(2):147–158
Matyas JR, Huang D, Chung M, Adams ME (2002) Regional quantification of cartilage type II collagen and aggrecan messenger RNA in joints with early experimental osteoarthritis. Arthritis Rheum 46(6):1536–1543
Matyas JR, Ehlers PF, Huang D, Adams ME (1999) The early molecular natural history of experimental osteoarthritis. I. Progressive discoordinate expression of aggrecan and type II procollagen messenger RNA in the articular cartilage of adult animals. Arthritis Rheum 42(5):993–1002
Aigner T, Bertling W, Stoss H, Weseloh G, von der Mark K (1993) Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest 91:829–837
Yagi R, McBurney D, Laverty D, Weiner S, Horton WE Jr (2005) Intrajoint comparisons of gene expression patterns in human osteoarthritis suggest a change in chondrocyte phenotype. J Orthop Res 23(5):1128–1138
Huebner JL, Hanes MA, Beekman B, TeKoppele JM, Kraus VB (2002) A comparative analysis of bone and cartilage metabolism in two strains of guinea pig with varying degrees of naturally occurring osteoarthritis. Ostearthritis Cartilage 10:758–767
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lahm, A., Mrosek, E., Spank, H. et al. Changes in content and synthesis of collagen types and proteoglycans in osteoarthritis of the knee joint and comparison of quantitative analysis with Photoshop-based image analysis. Arch Orthop Trauma Surg 130, 557–564 (2010). https://doi.org/10.1007/s00402-009-0981-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-009-0981-y