Abstract
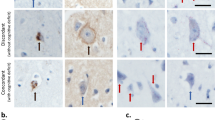
Mutations affecting proteolipid protein 1 (PLP1), the major protein in central nervous system myelin, cause the X-linked leukodystrophy Pelizaeus–Merzbacher disease (PMD). We describe the neuropathologic findings in a series of eight male PMD subjects with confirmed PLP1 mutations, including duplications, complete gene deletion, missense and exon-skipping. While PLP1 mutations have effects on oligodendrocytes that result in mutation-specific degrees of dysmyelination, our findings indicate that there are also unexpected effects in the central nervous system resulting in neuronal loss. Although length-dependent axonal degeneration has been described in PLP1 null mutations, there have been no reports on neuronal degeneration in PMD patients. We now demonstrate widespread neuronal loss in PMD. The patterns of neuronal loss appear to be dependent on the mutation type, suggesting selective vulnerability of neuronal populations that depends on the nature of the PLP1 disturbance. Nigral neurons, which were not affected in patients with either null or severe misfolding mutations, and thalamic neurons appear particularly vulnerable in PLP1 duplication and deletion patients, while hippocampal neuronal loss was prominent in a patient with complete PLP1 gene deletion. All subjects showed cerebellar neuronal loss. The patterns of neuronal involvement may explain some clinical findings, such as ataxia, being more prominent in PMD than in other leukodystrophies. While the precise pathogenetic mechanisms are not known, these observations suggest that defective glial functions contribute to neuronal pathology.



Similar content being viewed by others
References
Anderson TJ, Schneider A, Barrie JA et al (1998) Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene. J Comp Neurol 394:506–519
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Boison D, Stoffel W (1994) Disruption of the compacted myelin sheath of axons of the central nervous system in proteolipid protein-deficient mice. Proc Natl Acad Sci USA 91:11709–11713
Bond C, Si X, Crisp M et al (1997) Family with Pelizaeus–Merzbacher disease/X-linked spastic paraplegia and a nonsense mutation in exon 6 of the proteolipid protein gene. Am J Med Genet 71:357–360
Boucher SE, Cypher MA, Carlock LR, Skoff RP (2002) Proteolipid protein gene modulates viability and phenotype of neurons. J Neurosci 22:1772–1783
Carango P, Funanage VL, Quiros RE, Debruyn CS, Marks HG (1995) Overexpression of DM20 messenger RNA in two brothers with Pelizaeus–Merzbacher disease. Ann Neurol 38:610–617
Edgar JM, McLaughlin M, Yool D et al (2004) Oligodendroglial modulation of fast axonal transport in a mouse model of hereditary spastic paraplegia. J Cell Biol 166:121–131
Garbern JY, Cambi F, Tang XM et al (1997) Proteolipid protein is necessary in peripheral as well as central myelin. Neuron 19:205–218
Garbern JY, Yool DA, Moore GJ et al (2002) Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125:551–561
Gow A, Friedrich VL Jr, Lazzarini RA (1994) Many naturally occurring mutations of myelin proteolipid protein impair its intracellular transport. J Neurosci Res 37:574–583
Gow A, Lazzarini RA (1996) A cellular mechanism governing the severity of Pelizaeus–Merzbacher disease. Nat Genet 13:422–428
Griffiths I, Klugmann M, Anderson T et al (1998) Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280:1610–1613
Harding B, Ellis D, Malcolm S (1995) A case of Pelizaeus–Merzbacher disease showing increased dosage of the proteolipid protein gene. Neuropathol Appl Neurobiol 21:111–115
Hodes ME, Pratt VM, Dlouhy SR (1993) Genetics of Pelizaeus–Merzbacher disease. Dev Neurosci 15:383–394
Hudson LD, Puckett C, Berndt J, Chan J, Gencic S (1989) Mutation of the proteolipid protein gene PLP in a human X chromosome-linked myelin disorder. Proc Natl Acad Sci USA 86:8128–8131
Inoue K, Osaka H, Sugiyama N et al (1996) A duplicated PLP gene causing Pelizaeus–Merzbacher disease detected by comparative multiplex PCR. Am J Hum Genet 59:32–39
Inoue K, Osaka H, Thurston VC et al (2002) Genomic rearrangements resulting in plp1 deletion occur by nonhomologous end joining and cause different dysmyelinating phenotypes in males and females. Am J Hum Genet 71:838–853
Ip CW, Kroner A, Bendszus M et al (2006) Immune cells contribute to myelin degeneration and axonopathic changes in mice overexpressing proteolipid protein in oligodendrocytes. J Neurosci 26:8206–8216
Iwaki A, Muramoto T, Iwaki I et al (1993) A missense mutation in the proteolipid protein gene responsible for Pelizaeus–Merzbacher disease in a Japanese family. Hum Mol Genet 2:19–22
Kagawa T, Ikenaka K, Inoue Y et al (1994) Glial cell degeneration and hypomyelination caused by overexpression of myelin proteolipid protein gene. Neuron 13:427–442
Karim SA, Barrie JA, McCulloch MC et al (2007) PLP overexpression perturbs myelin protein composition and myelination in a mouse model of Pelizaeus–Merzbacher disease. Glia 55:341–351
Klugmann M, Schwab MH, Pühlhofer A et al (1997) Assembly of CNS myelin in the absence of proteolipid protein. Neuron 18:59–70
Koeppen AH, Robitaille Y (2002) Pelizaeus–Merzbacher disease. J Neuropathol Exp Neurol 61:747–759
Koeppen AH, Ronca NA, Greenfield EA, Hans MB (1987) Defective biosynthesis of proteolipid protein in Pelizaeus–Merzbacher disease. Ann Neurol 21:159–170
Mimault C, Giraud G, Courtois V et al (1999) Proteolipoprotein gene analysis in 82 patients with sporadic Pelizaeus–Merzbacher disease: duplications, the major cause of the disease, originate more frequently in male germ cells, but point mutations do not. The Clinical European Network on Brain Dysmyelinating Disease. Am J Hum Genet 65:360–369
Nave KA, Lai C, Bloom FE, Milner RJ (1987) Splice site selection in the proteolipid protein (PLP) gene transcript and primary structure of the DM-20 protein of central nervous system myelin. Proc Natl Acad Sci USA 84:5665–5669
Pelizaeus F (1885) Über eine eigenthümliche Form Spastischer Lähmung mit Cerebralerscheinungen auf hereditärer Grundlage (Multiple Sklerose). Arch Psychiatr Nervenkrankh 16:698–710
Pham-Dinh D, Birling MC, Roussel G, Dautigny A, Nussbaum JL (1991) Proteolipid DM-20 predominates over PLP in peripheral nervous system. Neuroreport 2:89–92
Pratt VM, Boyadjiev S, Green K, Hodes ME, Dlouhy SR (1995) Pelizaeus–Merzbacher disease caused by a de novo mutation that originated in exon 2 of the maternal great-grandfather of the propositus. Am J Med Genet 58:70–73
Raskind WH, Williams CA, Hudson LD, Bird TD (1991) Complete deletion of the proteolipid protein gene (PLP) in a family with X-linked Pelizaeus–Merzbacher disease. Am J Hum Genet 49:1355–1360
Readhead C, Schneider A, Griffiths I, Nave KA (1994) Premature arrest of myelin formation in transgenic mice with increased proteolipid protein gene dosage. Neuron 12:583–595
Rosenfeld J, Freidrich VL Jr (1983) Axonal swellings in jimpy mice: does lack of myelin cause neuronal abnormalities? Neuroscience 10:959–966
Saugier-Veber P, Munnich A, Bonneau D et al (1994) X-linked spastic paraplegia and Pelizaeus–Merzbacher disease are allelic disorders at the proteolipid protein locus. Nat Genet 6:257–262
Seitelberger F (1995) Neuropathology and genetics of Pelizaeus–Merzbacher disease. Brain Pathol 5:267–273
Shy ME, Hobson G, Jain M et al (2003) Schwann cell expression of PLP1 but not DM20 is necessary to prevent neuropathy. Ann Neurol 53:354–365
Simons M, Kramer EM, Macchi P et al (2002) Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus–Merzbacher disease. J Cell Biol 157:327–336
Sistermans EA, de Coo RF, de Wijs IJ, van Oost BA (1998) Duplication of the proteolipid protein gene is the major cause of Pelizaeus–Merzbacher disease. Neurology 50:1749–1754
Skoff RP, Bessert DA, Cerghet M et al (2004) The myelin proteolipid protein gene modulates apoptosis in neural and non-neural tissues. Cell Death Differ 11:1247–1257
Southwood CM, Garbern J, Jiang W, Gow A (2002) The unfolded protein response modulates disease severity in Pelizaeus–Merzbacher disease. Neuron 36:585–596
Trapp BD, Peterson J, Ransohoff RM et al (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285
van der Knaap MS, Valk J. (2005) Magnetic resonance of myelin, myelination, and myelin disorders. Springer, New York, p 1084
Watanabe I, Patel V, Goebel HH et al (1973) Early lesion of Pelizaeus–Merzbacher disease: electron microscopic and biochemical study. J Neuropathol Exp Neurol 32:313–333
Wilkus RJ, Farrell DF (1976) Electrophysiologic observations in the classical form of Pelizaeus–Merzbacher disease. Neurology 26:1042–1045
Yamamoto T, Nanba E, Zhang H et al (1998) Jimpymsd mouse mutation and connatal Pelizaeus–Merzbacher disease. Am J Med Genet 75:439–440
Yin X, Baek RC, Kirschner DA et al (2006) Evolution of a neuroprotective function of central nervous system myelin. J Cell Biol 172:469–478
Acknowledgments
The authors are especially grateful to the families who generously donated specimens for research. J.G. thanks the PMD Foundation, the National Institutes of Health (NS043783), the Children’s Research Center of Michigan, and the National Multiple Sclerosis Society (RG3204) for support. G.M.H. acknowledges the Nemours Foundation and National Institutes of Health grant P20 RR-020173-01 from the National Center for Research Resources. The authors also are grateful to the Alzheimer disease Research Center of the University of Washington for assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sima, A.A.F., Pierson, C.R., Woltjer, R.L. et al. Neuronal loss in Pelizaeus–Merzbacher disease differs in various mutations of the proteolipid protein 1. Acta Neuropathol 118, 531–539 (2009). https://doi.org/10.1007/s00401-009-0562-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0562-8