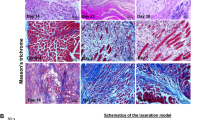
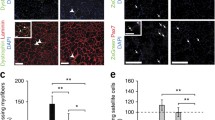
Abstract
The molecular signaling pathway linked to muscle regeneration has not yet been identified. Previously, we demonstrated that mice treated with cyclosporin A (CsA), a calcineurin inhibitor, failed to regenerate normally after muscle damage. Using reverse transcription (RT)-PCR, Western blot and immunohistochemical analysis, we investigated whether the amounts of nuclear factor of activated T cells (NFAT), myocyte-enhancer factor 2 (MEF2), the MyoD family, Id-1, and Smad3 change in the regenerating muscle after CsA treatment. Adult male ICR mice were subjected to a bupivacaine injection into the tibialis anterior muscle, and were treated with either CsA (25 mg/kg) or vehicle once daily. They were killed at 1, 2, 4, 6, 9 and 14 days post injury. RT-PCR analysis did not show a significant difference in MEF2s, MyoD and myogenin mRNA levels in the regenerating muscle in either placebo- and CsA-administered mice. In contrast, a significant increase in MRF4 mRNA was seen in CsA-administered mice compared to the placebo-treated mice at 4 and 9 days post surgery. In CsA-treated mice, the level of Id1 mRNA was elevated at day 9 relative to the placebo-treated mice. After 6 days, the CsA-treated mice possessed more abundant proliferating cell nuclear antigen (PCNA) and cyclin D1 protein in many satellite cells and/or myoblast-like cells in the regenerating muscle. The amount of myostatin, TGF-β2 and Smad3 mRNA and proteins was increased more markedly in the mice treated with CsA. After 9 days, many satellite cells and/or myoblasts showed apparent co-localization of both MyoD and Smad3 in CsA-, but not in placebo-, treated mice. Our results demonstrated that CsA treatment upregulates Id1 and Smad3 expression and delays skeletal muscle regeneration in vivo.









Similar content being viewed by others
References
Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK (1998) Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell 9:2905–2916
Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, McLafferty CL Jr, Urban RJ (2001) Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol 280:E383–E390
Bischoff R (1994) The satellite cell and muscle regeneration. In: Engel AG, Armstrong F (eds) Myology. McGraw-Hill, New York, pp 97–118
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Chakravarthy MV, Abraha TW, Schwartz RJ, Fiorotto ML, Booth FW (2000) Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of Phosphatidylinositol 3’-kinase/Akt signaling pathway. J Biol Chem 275:35942–35952
Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD (2000) A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol 20:6600–6611
Dunn SE, Burns JL, Michel RN (1999) Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem 274:21908–21912
Dunn SE, Chin ER, Michel RN (2000) Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J Cell Biol 151:663–672
Dunn SE, Simard AR, Bassel-Duby R, Williams RS, Michel RN (2001) Nerve activity-dependent modulation of calcineurin signaling in adult fast and slow skeletal muscle fibers. J Biol Chem 276:45243–45254
Engert JC, Berglund EB, Rosenthal N (1996) Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol 135:431–440
Fenyvesi R, Racz G, Wuytack F, Zador E (2004) The calcineurin activity and MCIP1.4 mRNA levels are increased by innervation in regenerating soleus muscle. Biochem Biophys Res Commun 320:599–605
Friday BB, Horsley V, Pavlath GK (2000) Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol 149:657–665
Friday BB, Mitchell PO, Kegley KM, Pavlath GK (2003) Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation 71:217–227
Gothel SF, Marahiel MA (1999) Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci 55:423–436
Guo K, Wang J, Andres V, Smith RC, Walsh K (1995) MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol 15:3823–3829
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Jen Y, Weintraub H, Benezra R (1992) Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev 8:1466–1479
Langlands K, Yin X, Anand G, Prochownik EV (1997) Differential interactions of Id proteins with basic-helix-loop-helix transcription factors. J Biol Chem 272:19785–19793
Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R (2002) Myostatin inhibits myoblast differentiation by downregulating MyoD expression. J Biol Chem 277:49831–49840
Lassar AB, Davis RL, Wright WE, Kadesch T, Murre C, Voronova A, Baltimore D, Weintraub H (1991) Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell 66:305–315
Liu D, Black BL, Derynck R (2001) TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 15:2950–2966
Mendler L, Zádor E, Dux L, Wuytack F (1998) mRNA levels of myogenic regulatory factors in rat slow and fast muscles regenerating from notexin-induced necrosis. Neuromuscul Disord 8:533–541
Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF (1999) Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22:724–732
Michel RN, Dunn SE, Chin ER (2004) Calcineurin and skeletal muscle growth. Proc Nutr Soc 63:341–349
Mitchell PO, Mills ST, Pavlath GK (2002) Calcineurin differentially regulates maintenance and growth of phenotypically distinct muscles. Am J Physiol Cell Physiol 282:C984–C992
Musaró A, McCullagh KJA, Naya FJ, Olson EN, Rosenthal N (1999) IGF-I induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400:581–585
Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S (2002) A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA 99:9213–9218
Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD (2004) Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem 279:26192–26200
Pavlath GK, Dominov JA, Kegley KM, Miller JB (2003) Regeneration of transgenic skeletal muscles with altered timing of expression of the basic helix-loop-helix muscle regulatory factor MRF4. Am J Pathol 162:1685–1691
Sakuma K, Watanabe K, Sano M, Sakamoto K, Uramoto I, Totsuka T(2000) The adaptive response of transforming growth factor-β2 and –βRII in the overloaded, regenerating and denervated muscles of rats. Acta Neuropathol 99:177–185
Sakuma K, Nishikawa J, Nakao R, Watanabe K, Totsuka T, Nakano H,Sano M, Yasuhara M (2003) Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathol 105:271–280
Sandri M, Sandri C, Brun B, Giurisato E, Cantini M, Rossini K, Destro C, Arsian P, Carraro U (2001) Inhibition of FasL sustains phagocytic cells and delays myogenesis in regenerating muscle fibers. J Leukoc Biol 69:482–489
Semsarian C, Wu M-J, Ju Y-K, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM (1999) Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 400:576–581
Sharma M, Langley B, Bass J, Kambadur R (2001) Myostatin in muscle growth and repair. Exerc Sport Sci Rev 29:155–158
Spiller MP, Kambadur R, Jeanplong F, Thomas M, Martyn JK, Bass JJ, Sharma M (2002) The myostatin gene is a downstream target gene of basic helix-loop-helix transcription factor MyoD. Mol Cell Biol 22:7066–7082
Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R (2002) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243
Xu Q, Yu L, Liu L, Cheung CF, Li X, Yee SP, Yang XJ, Wu Z (2002) p38 Mitogen-activated protein kinase-, calcium-calmodulin-dependent protein kinase-, and calcineurin-mediated signaling pathways transcriptionally regulate myogenin expression. Mol Biol Cell 13:1940–1952
Acknowledgements
This work was supported by a research Grant-in-Aid for Young Scientists B (nos. 13780029 and 15700423) from the Ministry of Education, Science, Sports and Culture of Japan.
Conflict of interest:
No information supplied
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakuma, K., Nakao, R., Aoi, W. et al. Cyclosporin A treatment upregulates Id1 and Smad3 expression and delays skeletal muscle regeneration. Acta Neuropathol 110, 269–280 (2005). https://doi.org/10.1007/s00401-005-1049-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-005-1049-x