Abstract
Aims
We report our experience concerning lead performance and re-surgery rate of the Medtronic EnRhythm MRI SureScan pacemaker system (MRI-PM) in comparison to standard pacemaker (PM) systems and leads used at our institution.
Methods
All patients (except patients with transvenous left ventricular leads) with successful PM implantation performed at our institution from 1 March 2009 to 31 October 2009 were included in this analysis and followed until mid January 2010. Lead measurements (assessed at implantation, prehospital discharge interrogation (1st follow-up) and at the first scheduled out-patient follow-up (2nd follow-up) were compared between atrial leads 4592–53 cm and 5086MRI–52 cm (lead group 1), and between ventricular leads 4092–58 cm and 5086MRI–52 cm/-58 cm (lead group 2), respectively. Causes for re-operations were assessed and compared between patients with standard dual chamber PM (DC-PM) and the MRI-PM.
Results
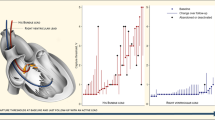
A total of 140 patients (VVI-PM: 36 patients; DDD-PM: 102 patients; biventricular PM: 1 patient) were successfully implanted with a PM within the implantation period. Two patients with transvenous left ventricular leads were excluded from further analysis. In an atrial position, lead 4592 was implanted in 51 patients and lead 5086MRI–52 cm was implanted in 40 patients, respectively. Ventricular leads were lead 4092–58 cm (64 patients) and lead 5086MRI (41 patients), respectively. Patients were followed for 26 ± 11 weeks. Comparison of lead measurements of lead group 1 showed significant differences for pacing impedance and pacing threshold at implantation, and for sensing at the 2nd follow-up. Comparison of lead measurements within lead group 2 showed significant differences for pacing impedance at implantation, for pacing threshold at the 1st follow-up, and for sensing, pacing threshold, and impedance at the 2nd follow-up. All assessed mean values were favorable for all leads at any follow-up. The number of re-operations was high in both dual chamber PM groups, but did not differ significantly between the two groups (DC-PM: 5 patients, 8.5%; MRI-PM: 5 patients, 13.2%).
Conclusion
Our study demonstrates favorable lead measurements of lead model 5086MRI in comparison to lead 4592 and 4092 in a short-term follow-up. The number of re-operations was higher in the MRI-PM group, but not statistically different in comparison with the standard dual chamber PM group.
Zusammnefassung
Hintergrund
Wir berichten unsere initiale Erfahrung hinsichtlich Elektrodenverhalten und Reoperationsrate des Medtronic EnRhythm MRI Sure Scan Schrittmachersystems im Vergleich zu den an unserer Abteilung benützten Standardschrittmachersystemen und -elektroden.
Methoden
Alle Patienten mit erfolgreicher Schrittmacherimplantation (außer jenen mit transvenösen linksventrikulären Elektroden) an unserer Abteilung zwischen 01.03.2009 und 31.10.2009 wurden in diese retrospektive Studie eingeschlossen und bis Mitte Januar 2010 nachbeobachtet. Elektrodenmesswerte (erhoben bei Implantation, vor Entlassung [1. Kontrolle] und bei der 4- bis 6-Wochen-Kontrolle [2. Kontrolle]) wurden verglichen zwischen den atrial implantierten Elektroden 4592–53 und 5086MRI–52 (Elektrodengruppe 1) und den ventrikulär implantierten Elektroden 4092–58 und 5086MRI–52/58 (Elektrodengruppe 2). Gründe für Reoperationen wurden ebenfalls erhoben und zwischen den Patienten mit Standard-DDD-Schrittmachern (DDD-SM) sowie den Patienten mit dem EnRhythm MRI SureScan Schrittmachersystem (MRI-SM) verglichen.
Ergebnisse
Im Implantationszeitraum wurde 140 Patienten erfolgreich ein Schrittmachersystem (VVI-SM: 36; DDD-SM: 102; biv. SM: 1) implantiert. Zwei Patienten mit transvenösen linksventrikulären Elektroden wurden von der weiteren Analyse ausgeschlossen. Rechtsatrial wurde das Elektrodenmodell 4592–53 bei 51 Patienten implantiert, das Modell 5086MRI–52 bei 40 Patienten. Elektrodenmodell 4092–58 wurde bei 64 Patienten rechtsventrikulär implantiert, Elektrodenmodell 5086MRI bei 41 Patienten. Die Nachbeobachtungszeit betrug 26 ± 11 Wochen. Beim Vergleich der Elektrodenmesswerte von Elektrodengruppe 1 zeigten sich signifikante Unterschiede für die Stimulationsimpedanz und Reizschwelle bei Implantation sowie für die Wahrnehmungsamplitude bei der 2. Kontrolle. Der Vergleich der Elektrodenmesswerte der Elektrodengruppe 2 zeigte signifikante Unterschiede für die Stimulationsimpedanz bei Implantation, für die Reizschwelle bei der 1. Kontrolle sowie für alle drei Parameter (Wahrnehmungsamplitude, Reizschwelle, Stimulationsimpedanz) bei der 2. Kontrolle. Insgesamt waren alle Elektrodenmesswerte bei jeder Kontrolle für alle Elektroden akzeptabel. Die Anzahl der Reoperationen war in beiden DDD-Schrittmachergruppen hoch, jedoch bestand kein signifikanter Unterschied (DDD-SM: 5 Patienten, 8,5%; MRI-SM: 5 Patienten, 13,2%).
Schlußfolgerung
Unsere Studie zeigt in dem kurzen Beobachtungszeitraum akzeptable Elektrodenmesswerte für die 5086MRI-Elektroden im Vergleich mit den Elektrodenmodellen 4595 und 4092. Die Anzahl der Reoperationen war in der MRI-SM-Gruppe zwar höher, der Unterschied war jedoch statistisch nicht signifikant.





Similar content being viewed by others
References
American College of Radiology (2011) ACR Practice Guidelines, http://www. acr.org. Accessed 20 Nov 2011
Roguin A, Schwitter J, Vahlhaus C et al (2008) Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace 10:336–346
Kalin R, Stanton MS (2005) Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 28:326–328
Sommer T, Naehle CP, Yang A et al (2006) Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 Tesla in the presence of cardiac pacemakers in non-pacemaker dependent patients. A prospective study with 115 examinations. Circulation 114:1285–1292
Nazarian S, Roguin A, Zviman MM et al (2006) Clinicial utility of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 Tesla. Circulation 114:1277–1284
Vardas PE, Auricchio A, Blanc JJ et al (2007) Guidelines for cardiac pacing and cardiac resynchronization therapy. The task force for cardiac pacing and cardiac resynchronization therapy of the European society of cardiology. Developed in collaboration with the European heart rhythm association. Eur Heart J 28:2256–2295
Epstein AE, DiMarco JP, Ellenbogen KA et al (2008) American college of cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American college of cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 guideline update for implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 51(21):e1–e62
Surescan™ (2008) Technical manual. Medtronic Inc (http://www.medtronic.com/manuals). Accessed 20 Nov 2011
ENRHYTHM MRI™ SURESCAN EMDR01 (2008) Reference manual. Medtronic Inc (http://www.medtronic.com/manuals). Accessed 20 Nov 2011
CAPSUREFIX MRI™ SURESCAN™ 5086MRI (2009) Manual. Medtronic Inc (http://www.medtronic.com/manuals). Accessed 20 Nov 2011
Medtronic News Release 2008. http://www.medtronic.com. Accessed 20 Nov 2011
Nitschke T, Heuer A, Scheffold T, Heuer H (2009) In-Vitro-Vergleich der Temperaturentwicklung einer MRT-tauglichen Schrittmachersonde im Vergleich mit einer konventionellen Schrittmachersonde im 1,5 Tesla Magnetresonanztomographen. Clin Res Cardiol 98(Suppl 2)
Wilkoff BL, Bello D, Taborsky M et al (2011) Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm 8:65–73
Kirkfeldt RE, Johansen JB, Nohr EA et al (2011) Risk factors for lead complications in cardiac pacing: a population-based cohort study of 28,860 Danish Patients. Heart Rhythm 8:1622–1628
Luria DM, Feinberg MS, Gurevitz OT et al (2007) Randomized comparison of J-shaped atrial leads with and without active fixation mechanism. Pacing Clin Electrophysiol 30:412–417
Ellenbogen KA, Hellkamp AS, Wilkoff BL et al (2003) Complications arising after implantation of DDD pacemakers: the MOST experience. Am J Cardiol 92(6):740–741
Glikson M, Hyberger LK, Hitzke MK et al (1999) Clinical surveillance of a tined, bipolar, J-shaped, steroid-eluting, silicone-insulated atrial pacing lead. Pacing Clin Electrophysiol 22:1079–1081
Fleck T, Khazen C, Wolner E, Grabenwöger M (2006) The incidence of reoperations in pacemaker recipients. Heart Surg Forum 2006
Markewitz A (2009) Annual report 2007 of the German pacemaker registry. Expert group pacemakers and the National Institute for Quality in Health Care (BQS Bundesgeschäftsstelle Qualitätssicherung gGmbH; managing director: Dr. C. Veit), Düsseldorf. Herzschrittmacherther Elektrophysiol 20:191–218
DPR (2011) Danish Pacemaker Register. Department of Cardiology, Odense University Hospital, Denmark (http://www.pacemaker.dk)
Conflict of interest
The corresponding author states the following: Dr. C.G. Wollmann: Biotronik, Boston Scientific, St. Jude Medical (consulting); Dr. H. Mayr: Medtronic (consulting).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wollmann, C., Thudt, K., Vock, P. et al. Clinical routine implantation of a dual chamber pacemaker system designed for safe use with MRI. Herzschr. Elektrophys. 22, 233–242 (2011). https://doi.org/10.1007/s00399-011-0161-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00399-011-0161-y