Abstract
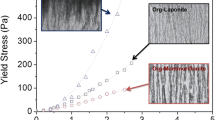
Montmorillonite dispersions were completely coagulated ¶by magnesium aluminum hydroxide when the hydroxide mass fraction, χ, was 0.2 or greater. The hydroxide dispersion required only a montmorillonite mass fraction of 0.06 for total coagulation. Thus, heterocoagulates were formed for 0.2<χ<0.94. At an excess of montmorillonite, network formation between the oppositely charged particles led to maxima of the yield value and the storage modulus at 0.4<χ<0.5. At higher hydroxide contents, χ>0.5, both properties decreased steeply, indicating the reduced mechanical stability of the network. Divalent anions at concentrations above 1 mmol/l acted as liquefying agents for the dispersed heterocoagulates. The specific surface area of the freeze-dried dispersions increased to a maximum value at χ = 0.65. The pore size distributions revealed that montmorillonite lamellae and hydroxide particles were not homogeneously distributed.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 28 February 2001 Accepted: 8 March 2001
Rights and permissions
About this article
Cite this article
Lagaly, G., Mecking, O. & Penner, D. Colloidal magnesium aluminum hydroxide and heterocoagulation with a clay mineral. II. Heterocoagulation with sodium montmorillonite. Colloid Polym Sci 279, 1097–1103 (2001). https://doi.org/10.1007/s003960100526
Issue Date:
DOI: https://doi.org/10.1007/s003960100526