Abstract
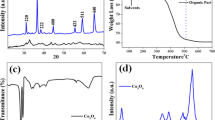
We design and prepare porous N-doped carbon sub-microspheres (Por-N–C-SS) using the phenolic resin sub-microspheres as precursor and potassium hydroxide as pore forming agent by polymerization-carbonization strategy. Pt and Cu ions are impregnated on surface of Por-N–C-SS, and then PtCu/Por-N–C-SS are obtained by a facile reduction process. The as-fabricated Por-N–C-SS exhibits the spherical structure with the diameter size of 200–500 nm. And Por-N–C-SS also possesses high specific surface area of 2222 m2 g−1 and large pore volume of 1.02 cm3 g−1. Benefiting from alloy effect of highly dispersed PtCu and N-doped porous carbon architecture, PtCu/Por-N–C-SS shows excellent electrocatalytic performance toward methanol oxidation reaction with high peak current density (4661 mA cm−2 mg−1 Pt) and large Jf/Jb value (1.18), exceeding commercial Pt/C catalyst in acid media. Furthermore, PtCu/Por-N–C-SS also reveals an outstanding catalytic stability, promoting its practical application in fuel cells.






Similar content being viewed by others
References
Debe MK (2012) Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486:43. https://doi.org/10.1038/nature11115
Xia Z, Guo S (2019) Strain engineering of metal-based nanomaterials for energy electrocatalysis. Chem Soc Rev 48(12):3265–3278. https://doi.org/10.1039/C8CS00846A
Xu D, Liu Z, Yang H, Liu Q, Zhang J, Fang J, Zou S, Sun K (2009) Solution-based evolution and enhanced methanol oxidation activity of monodisperse platinum–copper nanocubes. Angew Chem Int Ed 48(23):4217–4221. https://doi.org/10.1002/anie.200900293
Cui X, Xiao P, Wang J, Zhou M, Guo W, Yang Y, He Y, Wang Z, Yang Y, Zhang Y, Lin Z (2017) Highly branched metal alloy networks with superior activities for the methanol oxidation reaction. Angew Chem Int Ed 56(16):4488–4493. https://doi.org/10.1002/anie.201701149
Zhu QL, Tsumori N, Xu Q (2015) Immobilizing extremely catalytically active palladium nanoparticles to carbon nanospheres: a weakly-capping growth approach. J Am Chem Soc 137(36):11743–11748. https://doi.org/10.1021/jacs.5b06707
Tian H, Yu Y, Wang Q, Li J, Rao P, Li R, Du Y, Jia C, Luo J, Deng P, Shen Y, Tian X (2021) Recent advances in two-dimensional Pt based electrocatalysts for methanol oxidation reaction. Int J Hydrogen Energy 46(61):31202–31215. https://doi.org/10.1016/j.ijhydene.2021.07.006
Chen L, Zhou L, Lu H, Zhou Y, Huang J, Wang J, Wang Y, Yuan X, Yao Y (2020) Shape-controlled synthesis of planar PtPb nanoplates for highly efficient methanol electro-oxidation reaction. Chem Commun 56(64):9138–9141. https://doi.org/10.1039/D0CC03704D
Chen Y, Yang J, Yang Y, Peng Z, Li J, Mei T, Wang J, Hao M, Chen Y, Xiong W, Zhang L, Wang X (2015) A facile strategy to synthesize three-dimensional Pd@Pt core-shell nanoflowers supported on graphene nanosheets as enhanced nanoelectrocatalysts for methanol oxidation. Chem Commun 51(52):10490–10493. https://doi.org/10.1039/C5CC01803J
Li S, Tian ZQ, Liu Y, Jang Z, Hasan SW, Chen X, Tsiakaras P, Shen PK (2021) Hierarchically skeletal multi-layered Pt-Ni nanocrystals for highly efficient oxygen reduction and methanol oxidation reactions. Chin J Catal 42(4):648–657. https://doi.org/10.1016/S1872-2067(20)63680-4
Zhang J, Li K, Zhang B (2015) Synthesis of dendritic Pt-Ni-P alloy nanoparticles with enhanced electrocatalytic properties. Chem Commun 51(60):12012–12015. https://doi.org/10.1039/C5CC04277A
Zhang B, Niu Y, Xu J, Pan X, Chen C-M, Shi W, Willinger M-G, Schlogl R, Su DS (2016) Tuning the surface structure of supported PtNix bimetallic electrocatalysts for the methanol electro-oxidation reaction. Chem Commun 52(20):3927–3930. https://doi.org/10.1039/C5CC08978F
Eid K, Wang H, He P, Wang K, Ahamad T, Alshehri SM, Yamauchi Y, Wang L (2015) One-step synthesis of porous bimetallic PtCu nanocrystals with high electrocatalytic activity for methanol oxidation reaction. Nanoscale 7(40):16860–16866. https://doi.org/10.1039/C5NR04557F
Xia BY, Wu HB, Wang X, Lou XW (2012) One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J Am Chem Soc 134(34):13934–13937. https://doi.org/10.1021/ja3051662
Wang Y-X, Zhou H-J, Sun P-C, Chen T-H (2014) Exceptional methanol electro-oxidation activity by bimetallic concave and dendritic Pt–Cu nanocrystals catalysts. J Power Sources 245:663–670. https://doi.org/10.1016/j.jpowsour.2013.07.015
Byun J, Ahn SH, Kim JJ (2020) Self-terminated electrodeposition of platinum on titanium nitride for methanol oxidation reaction in acidic electrolyte. Int J Hydrogen Energy 45(16):9603–9611. https://doi.org/10.1016/j.ijhydene.2020.01.204
Musthafa OTM, Sampath S (2008) High performance platinized titanium nitride catalyst for methanoloxidation. Chem Comms (1):67–69. https://doi.org/10.1039/B715859A
Liu Y, Yan H, Zhou X, Li M, Fu H (2015) Small-sized tungsten nitride particles strongly anchored on carbon nanotubes and their use as supports for Pt for methanol electro-oxidation. Chem – A Eur J 21(50):18345–18353. https://doi.org/10.1002/chem.201503150
Wang P, Kottakkat T, Bron M (2015) Pt supported on nanostructured NCNTs/RGO composite electrodes for methanol electrooxidation. ChemElectroChem 2(9):1396–1402. https://doi.org/10.1002/celc.201500044
Li J, Zhu Q-L, Xu Q (2015) Pd nanoparticles supported on hierarchically porous carbons derived from assembled nanoparticles of a zeolitic imidazolate framework (ZIF-8) for methanol electrooxidation. Chem Commun 51(54):10827–10830. https://doi.org/10.1039/C5CC03008K
Liu Y-L, Shi C-X, Xu X-Y, Sun P-C, Chen T-H (2015) Nitrogen-doped hierarchically porous carbon spheres as efficient metal-free electrocatalysts for an oxygen reduction reaction. J Power Sources 283:389–396. https://doi.org/10.1016/j.jpowsour.2015.02.151
Li W, Min C, Tan F, Li Z, Zhang B, Si R, Xu M, Liu W, Zhou L, Wei Q, Zhang Y, Yang X (2019) Bottom-up construction of active sites in a Cu–N4–C catalyst for highly efficient oxygen reduction reaction. ACS Nano 13(3):3177–3187. https://doi.org/10.1021/acsnano.8b08692
Wang M, Gao P, Li D, Wu X, Yang M, Li Z, Shen Y, Hu X, Liu Y, Chen Z (2022) Cu/Fe dual atoms catalysts derived from Cu-MOF for Zn-air batteries. Mater Today Energy 28:101086. https://doi.org/10.1016/j.mtener.2022.101086
Wasmus S, Küver A (1999) Methanol oxidation and direct methanol fuel cells: a selective review1In honour of Professor W. Vielstich on the occasion of his 75th birthday and in appreciation of his contributions to electrochemistry as well as fuel cell development.1. J Electroanal Chem 461(1):14–31. https://doi.org/10.1016/S0022-0728(98)00197-1
Funding
We would like to acknowledge the financial supports from the Natural Science Foundation of Jiangsu Province of China (BK20160982).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, B., Qiu, T., Zhou, H. et al. Porous N-doped carbon sub-microspheres with PtCu nanoparticles for efficient methanol electro-oxidation. Colloid Polym Sci 301, 623–629 (2023). https://doi.org/10.1007/s00396-023-05098-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-023-05098-x