Abstract
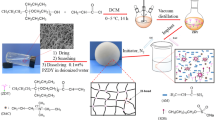
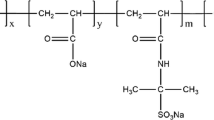
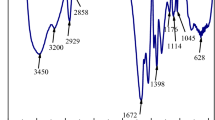
Polyacrylamide (HPAM) is commonly used as a thickener in water-based fracturing fluids due to its good solubility and thickening ability. However, drawbacks such as the formation of high temperature and high salinity in oil and gas production currently limit its use as a thickening agent for fracturing fluids. To solve this problem, a hydrophobic associating polymer, DSAM (acrylamide/2-acrylamido-2-methylpropanesulfonic acid/acrylic acid/hydrophobic monomer AMD-12), with a good temperature and salt resistance was synthesized via complex initiated polymerization. The molecular structure of the synthesized polymer DSAM was confirmed using IR and 1H NMR. The water solubility, thickening properties, and salt resistance of DSAM polymers were investigated. The results showed that the DSAM polymer solution’s apparent viscosity initially decreased with the addition of NaCl. However, as the salt concentration further increased, the DSAM polymer solution’s polarity also increased, as well as the hydrophobic association between molecules, resulting in a denser hydrophobic association network structure and an increase in the apparent viscosity of the polymer solution. The viscoelasticity test revealed that as the salt concentration increased, the viscoelastic polymer solution increased after initially decreasing, which was consistent with previous salt tolerance test results. Additionally, it exhibited superior temperature resistance, shear tolerance, and shear recovery capabilities compared with conventional HPAM. Meanwhile, the DSAM polymer can be completely broken down in the industry-standard time without residue. The benefits of DSAM polymers include salt thickening, high-temperature resistance, and thorough gel breaking. Thus, it has huge potential as a thickening agent for temperature-tolerant and salt-resistant fracturing fluid.













Similar content being viewed by others
Change history
18 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00396-022-04991-1
References
Montgomery CT, Smith MB (2015) Hydraulic fracturing: history of an enduring technology. J Petrol Technol 62:26–40
Palisch TT, Vincent M, Handren PJ (2010) Slickwater fracturing: food for thought. SPE Prod Oper 25:327–344
Reza B, And J-T, Liang, (2014) A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J Appl Polym Sci 131:318–323
Guan BS, Wang YF, Wu HZ, Biao DU, Liu J (2006) Development of CJ2–3 type recoverable low molecular mass guar gum fracturing fuid. Oilfield Chem
Chowdhary MS, Cottrell IW (1999) Method for preparation of amphoteric guar gum derivatives. EP
Fan H, Zhang Y, Fang Y, Yang F (2014) Research and application on low-molecular polymer fracturing fluid in Tahe oilfield. Drill Fluid & Completion Fluid
Tao DU, Yao YM, Jiang TX, Zhang XD, Peng MA, Nie YZ (2015) Study of rheological property and proppant-carrying capacity for reversible cross-linking polymer fracturing fluid. Appl Chem Indus
Holtsclaw J, Funkhouser GP (2010) A crosslinkable synthetic-polymer system for high-temperature hydraulic-fracturing applications. SPE Drill Complet 25:555–563
Guo YJ, Liu JX, Zhang XM, Feng RS, Li HB, Jian Z et al (2012) Solution property investigation of combination flooding systems consisting of gemini-non-ionic mixed surfactant and hydrophobically associating polyacrylamide for enhanced oil recovery. Energy Fuels 26:2116–2123
Dai LY (2015) Synthesis and performance evaluation of AM/AMPS/DM-16 hydrophobically associating polymer fracturing fluid thickener [D]. Suthwest Petroleum University, Chengdu
Li X, Xu Z, Yin H, Feng Y, Quan H (2017) Comparative studies on enhanced oil recovery: thermoviscosifying polymer versus polyacrylamide. Energy Fuels 31:2479–2487
Gou S, Yin T, Ye Z, Jiang W, Yang C, Ma Y et al (2014) High‐temperature resistance water‐soluble copolymer derived from acrylamide, DMDAAC, and functionalized sulfonamide for potential application in enhance oil recovery. J Appl Polym ence 131:n/a-n/a
Seright RS, Campbell A, Mozley P (2010) Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent ctions. SPE J 15:341–348
Zhang Y, Mao J, Zhao J, Yang X, Xu T, Lin C et al (2019) Preparation of a hydrophobic-associating polymer with ultra-high salt resistance using synergistic efect. Polymers 11
Uranta KG, Rezaei-Gomari S, Russell PFH (2018) Studying the effectiveness of polyacrylamide (PAM) application in hydrocarbon reservoirs at dfferent operational conditions. Energies 11
Pu WF, Liu R, Wang KY, Li KX, Lei Z (2015) Water-soluble core-shell hyperbranched polymers for enhanced oil recovery. Ind Eng Chem Res 54:798–807
Xiaowu Y, Yiding S, Peizhi L (2010) Solution properties of anionic hydrophobic association polyacrylamide modified with fluorinated acrylate. J Polym Res 17:601–606
Xue W, Hamley IW, Castelletto V, Olmsted PD (2004) Synthesis and characterization of hydrophobically modified polyacrylamides and some observations on rheological properties. Eur Polymer J 40:47–56
Smith GL, Mccormick CL (2001) Water-soluble polymers. 80. rheological and photophysical studies of pH-responsive terpolymers containing hydrophobic twin-tailed acrylamide monomers. Macromolecules 34
Feng ZQ, Xin W, Kong Y, Wang YF, Yang JR, Hou YF (2004) Study on hydrophobically associating AM/DBA copolymer for tertiary oil recovery. Applied Chemistry 21:556–560
Li JZ, Pu WF, Yang Y (2011) Synthesis and performance of AM/AMPS/third monomer terpolymer temperature-resistant and salt-resistant oil displacement system. Journal of Petroleum and Natural Gas 33:4
Tang H, Li JB, Liu L, Liao L (2007) Synthesis and evaluation of temperature-resistant and salt-resistant AM/AA/AMPS/DMDAAC copolymers. Advances in Fine Petrochemicals 8:4
Jain S, Prestridge AR, Dellorusso P, Nguyen NC, Hung V (2007) Case study from 12 successful years of high temperature fracturing in Bach Ho field offshore Vietnam
Zhang Y, Mao J, Zhao J, Yang X, Zhang H (2018) Preparation of a novel ultra-high temperature low-damage fracturing fluid system using dynamic crosslinking strategy. Chem Engi J 354
Yin X (2010) Design, synthesis and solution properties of a new temperature-resistant and salt-resistant polymer for oil and gas exploitation
Yu ZS, Xia YM, Li YC (2012) Newest research development of acrylamide based polymer flooding agent with temperature resistance and salt tolerance. Fine Chemicals
Peng B, Peng S, Long B, Miao Y, Guo WY (2010) Properties of high-temperature-resistant drilling fluids incorporating acrylamide/(acrylic acid)/(2-acrylamido-2-methyl-1-propane sulfonic acid) terpolymer and aluminum citrate as filtration control agents. J Vinyl Addit Technol
Bo L (2015) Synthesis and performance evaluation on a new type of salt resistant polymer. J Yangtze Univ (Natural Science Edition)
Zhang Y (2018) Preparation and properties of a high-temperature fracturing fluid thickener [D]. Southwest Petroleum University, Chengdu
Baker JP, Stephens DR, Blanch HW, Prausnitz JM (1991) Swelling equilibria for acrylamide-based polyampholyte hydrogels. Macromolecules 25:1955–1958
Ran W, Song W, F Y, Zhou J, Man Z, Zhang X et al (2018) Bidirectionally pH-responsive zwitterionic polymer hydrogels with switchable selective adsorption capacities for anionic and cationic dyes. Indus Eng Chem Res 57:acs.iecr.8b01027
Zhang J (1999) Hydrophobic cation monomer ADMCAB and property of aqueous solution for its copolymers with AM. Acta Entlarum Naturalium Universitatis Sunyatseni
Sigitov VB, Salina AA, Kudaibergenov SE, Nurkeeva ZS, Shaikhutdinov EM (1999) Oscillatory relaxation properties of polyampholyte hydrogels
Zhang. L. (1998) Zwitterionic polymers with antipolyelectrolyte behavior in solution. Polym Bullet
Chen X, Huang F (2009) Research development of heat-resistant and salt-tolerant polymer for enhanced oil recovery. Petrochem Technol
Rabiee A, Ershad-Langroudi A, Jamshidi H (2014) Polyacrylamide-based polyampholytes and their applications. Rev Chem Eng 30:501–519
Zhang Y, Yin H, Feng Y (2019) Opposite-charged polyelectrolyte complexes for high-salinity hydrofracking fluid. Indus Eng Chem Res 58
Kang W, Fan HAimming A, Saule B et al (2017) Study of salt tolerance and temperature resistance of a hydrophobically modified polyacrylamide based novel functional polymer for EOR. Colloids and Surfaces, A. Physicochemical and Eng Aspec
Pu WF, Yang Y, Wei B, Yuan CD (2016) The potential of a β-cyclodextrin/adamantane modified copolymer in enhancing oil recovery through host-guest interactions. Ind Eng Chem Res 55:8679–8689
Funding
The research is partly supported by the National Natural Science Foundation of Chian (Grant No. 41902303), China Postdoctoral Science Foundation (2019M650250), and the National High Technology Research & Development Program (2016ZX05053, 2016ZX05014-005–007).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The experiment image labels of Fig. 11 of this article are incorrect; the correct figure labels should have appeared as shown below.
Rights and permissions
About this article
Cite this article
Mao, J., Xue, J., Zhang, H. et al. Investigation of a hydrophobically associating polymer’s temperature and salt resistance for fracturing fluid thickener. Colloid Polym Sci 300, 569–582 (2022). https://doi.org/10.1007/s00396-022-04965-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-022-04965-3