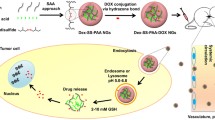
Abstract
A novel strategy for the preparation of dual-responsive prodrug nanogels was proposed by Diels-Alder reaction. Firstly, poly(styrene-alt-maleic anhydride) copolymers were functionalized with furfuryl amine and hydrazine in a simple one-pot reaction. Secondly, doxorubicin (DOX) was further incorporated to copolymer via hydrazone linkage in the presence of dithiobismaleimidoethane as a cross-linking agent resulted in prodrug nanogels. The prodrug nanogels were stable under physiological condition while the disintegration of nanogels was taken place and DOX released rapidly in the redox medium. The prodrug nanogels presented strong toxicity towards HepG2 cell line, whereas they had almost no inhibition in HEK293 normal cell line. The results suggest that DOX conjugate-based nanogels would be a potential candidate for efficient drug nanocarrier.

Graphical abstract









Similar content being viewed by others
References
Wang Y, Liu Y, Liu Y, Zhou W, Wang H, Wan G, Sun D, Zhang N, Wang Y (2015) A polymeric prodrug of cisplatin based on pullulan for the targeted therapy against hepatocellular carcinoma. Int J Pharm 483:89–100. https://doi.org/10.1016/j.ijpharm.2015.02.027
Singh SVB, Jung E, Noh J, Yoo D, Kang C, Hyeon H, Kim G-W, Khang G, Lee D (2019) Hydrogen peroxide-activatable polymeric prodrug of curcumin for ultrasound imaging and therapy of acute liver failure. Nanomed Nanotechnol 16:45–55. https://doi.org/10.1016/j.nano.2018.11.003
Horo H, Das S, Mandal B, Kundu LM (2019) Development of a photoresponsive chitosan conjugated prodrug nanocarrier for controlled delivery of antitumor drug 5-fluorouracil. Int J Biol Macromol 121:1070–1076. https://doi.org/10.1016/j.ijbiomac.2018.10.095
Kim DW, Cao XT, Kim YH, Gal Y-S, Lim KT (2017) Block copolymeric micelles of poly(ethylene oxide)-b-poly (glycidyl methacrylate) for pH triggered drug release. Mol Cryst Liq Cryst 644:145–151. https://doi.org/10.1080/15421406.2016.1278131
Cao XT, Kim YH, Park JM, Lim KT (2016) One-pot syntheses of dual responsive core cross-linked polymeric micelles and covalently entrapped drug by click chemistry. Eur Polym J 78:264–273. https://doi.org/10.1016/j.eurpolymj.2016.03.039
Li D, Song Y, He J, Zhang M, Ni P (2019) Polymer-doxorubicin prodrug with biocompatibility, pH response, and main chain breakability prepared by catalyst-free click reaction. ACS Biomater Sci Eng 5:2307–2315. https://doi.org/10.1021/acsbiomaterials.9b00301
Kaur K, Jindal R (2020) Comparative studies of directly loaded and cyclodextrin-mediated release of theophylline and evaluation of biodegradation studies of HPNs. Polym Bull. https://doi.org/10.1007/s00289-020-03323-z
Sonawane SJ, Kalhapure RS, Govender T (2017) Hydrazone linkages in pH responsive drug delivery systems. Eur J Pharm Sci 99:45–65. https://doi.org/10.1016/j.ejps.2016.12.011
Cao XT, Le CMQ, Thi HHP, Kim G-D, Gal Y-S, Lim KT (2017) Redox-responsive core cross linked prodrug micelles prepared by click chemistry for pH-triggered doxorubicin delivery. Express Polym Lett 11:832–845. https://doi.org/10.3144/expresspolymlett.2017.79
Sirova M, Mrkvan T, Etrych T, Chytil P, Rossmann P, Ibrahimova M, Kovar L, Ulbrich K, Rihova B (2010) Preclinical evaluation of linear HPMA-doxorubicin conjugates with pH-sensitive drug release: efficacy, safety, and immunomodulating activity in murine model. Pharm Res 27:200–208. https://doi.org/10.1007/s11095-009-9999-7
Vetvicka D, Hruby M, Hovorka O, Etrych T, Vetrik M, Kovar L, Kovar M, Ulbrich K, Rihova B (2009) Biological evaluation of polymeric micelles with covalently bound doxorubicin. Bioconjug Chem 20:2090–2097. https://doi.org/10.1021/bc900212k
Coimbra M, Rijcken CJF, Stiger M, Hennink WE, Storm G, Schiffelers RM (2012) Antitumor efficacy of dexamethasone-loaded core-crosslinked polymeric micelles. J Control Release 163:361–367. https://doi.org/10.1016/j.jconrel.2012.09.014
Talelli M, Iman M, Varkouhi AK, Rijcken CJF, Schiffelers RM, Etrych T, Ulbrich K, van Nostrum CF, Lammers T, Storm G, Hennink WE (2010) Core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin. Biomaterials 31:7797–7804. https://doi.org/10.1016/j.biomaterials.2010.07.005
Etrych T, Mrkvan T, Chytil P, Konak C, Rihova B, Ulbrich K (2008) N- (2-hydroxypropyl)methacrylamide-based polymer conjugates with pH-controlled activation of doxorubicin. I. New synthesis, physicochemical characterization and preliminary biological evaluation. J Appl Polym Sci 109:3050–3061. https://doi.org/10.1002/app.28466
Elkassih SA, Kos P, Xiong H, Siegwart DJ (2019) Degradable redox-responsive disulfide based nanogel drug carriers via dithiol oxidation polymerization. Biomater Sci 7:607–617. https://doi.org/10.1039/C8BM01120F
Nikfarjam M, Kokabi M (2020) Chitosan/laponite nanocomposite nanogels as a potential drug delivery system. Polym Bull. https://doi.org/10.1007/s00289-020-03335-9
Oehrl A, Schötz S, Haag R (2020) Synthesis of pH-degradable polyglycerol-based nanogels by iEDDA-mediated crosslinking for encapsulation of asparaginase using inverse nanoprecipitation. Colloid Polym Sci 298:719–733. https://doi.org/10.1007/s00396-020-04675-8
Ashrafizadeh M, Tam KC, Javadi A, Abdollahi M, Sadeghnejad S, Bahramian A (2019) Synthesis and physicochemical properties of dual-responsive acrylic acid/butyl acrylate cross-linked nanogel systems. J Colloid Interface Sci 556:313–323. https://doi.org/10.1016/j.jcis.2019.08.066
Baranello MP, Bauer L, Benoit DSW (2014) Poly(styrene-alt-maleic anhydride)-based diblock copolymer micelles exhibit versatile hydrophobic drug loading, drug dependent release, and internalization by multidrug resistant ovarian cancer cells. Biomacromolecules 15:2629–2641. https://doi.org/10.1021/bm500468d
Li Y, Li P, Lu J, Zhao Y (2020) Synthesis of pH-, thermo- and salt-responsive hydrogels containing MCM-41 as crosslinker in situ for controlled drug release. Polym Bull. https://doi.org/10.1007/s00289-020-03325-x
Saidi M, Dabbaghi A, Rahmani S (2020) Swelling and drug delivery kinetics of click-synthesized hydrogels based on various combinations of PEG and star-shaped PCL: influence of network parameters on swelling and release behavior. Polym Bull 77:3989–4010. https://doi.org/10.1007/s00289-019-02948-z
Zhang X, Malhotra S, Molina M, Haag R (2015) Micro- and nanogels with labile crosslinks – from synthesis to biomedical applications. Chem Soc Rev 44:1948–1973. https://doi.org/10.1039/C4CS00341A
Sivaram AJ, Rajitha P, Maya S, Jayakumar R, Sabitha M (2015) Nanogels for delivery, imaging and therapy. WIREs Nanomed Nanobi 7:509–533. https://doi.org/10.1002/wnan.1328
Wang Q, Gao F, Zhou X (2020) Redox-responsive AIE micelles for intracellular paclitaxel delivery. Colloid Polym Sci 298:1119–1128. https://doi.org/10.1007/s00396-020-04679-4
Li M, Tang Z, Sun H, Ding J, Song W, Chen X (2013) pH and reduction dual-responsive nanogel cross-linked by quaternization reaction for enhanced cellular internalization and intracellular drug delivery. Polym Chem 4:1199–1207. https://doi.org/10.1039/C2PY20871G
Sisson AL, Papp I, Landfester K, Haag R (2009) Functional nanoparticles from dendritic precursors: hierarchical assembly in miniemulsion. Macromolecules 42:556–559. https://doi.org/10.1021/ma802238e
Chen W, Achazi K, Schade B, Haag R (2015) Charge-conversional and reduction-sensitive poly(vinyl alcohol) nanogels for enhanced cell uptake and efficient intracellular doxorubicin release. J Control Release 205:15–24. https://doi.org/10.1016/j.jconrel.2014.11.012
Steinhilber D, Sisson AL, Mangoldt D, Welker P, Licha K, Haag R (2010) Synthesis, reductive cleavage and cellular interaction studies of biodegradable, polyglycerol nanogels. Adv Funct Mater 20:4133–4138. https://doi.org/10.1002/adfm.201000410
Zhang Y, Ding J, Li M, Chen X, Xiao C, Zhuang X, Huang Y, Chen X (2016) One-step “click chemistry”-synthesized cross-linked prodrug nanogel for highly selective intracellular drug delivery and upregulated antitumor efficacy. ACS Appl Mater Interfaces 8:10673–10682. https://doi.org/10.1021/acsami.6b00426
Yamamoto S, Kaneo Y, Maeda H (2013) Styrene maleic acid anhydride copolymer (SMA) for the encapsulation of sparingly water-soluble drugs in nanoparticles. J Drug Del Sci Tech 23:231–237. https://doi.org/10.1016/S1773-2247(13)50035-9
Padmavathy N, Samantaray PK, Ghosh LD, Madras G, Bose S (2017) Selective cleavage of polyphosphoester in crosslinked copper based nanogels: enhanced antibacterial performance through controlled release of copper. Nanoscale 9:12664–12676. https://doi.org/10.1039/C7NR02446K
Maeda H, Ueda M, Morinaga T, Matsumoto T (1985) Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J Med Chem 28:455–461. https://doi.org/10.1021/jm00382a012
Chacko RT, Ventura J, Zhuang J, Thayumanavan S (2012) Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv Drug Deliv Rev 64:836–851. https://doi.org/10.1016/j.addr.2012.02.002
Le CMQ, Cao XT, Lim KT (2017) Ultrasound-promoted direct functionalization of multi-walled carbon nanotubes in water via Diels-Alder “click chemistry”. Ultrason Sonochem 39:321–329. https://doi.org/10.1016/j.ultsonch.2017.04.042
Le CMQ, Cao XT, Tu TTK, Gal Y-S, Lim KT (2018) Facile approach to prepare pH and redox responsive nanogels via Diels-Alder click reaction. Express Polym Lett 12:688–698. https://doi.org/10.3144/expresspolymlett.2018.59
Zhan F, Chen W, Wang Z, Lu W, Cheng R, Deng C, Meng F, Liu H, Zhong Z (2011) Acid-activatable prodrug nanogels for efficient intracellular doxorubicin release. Biomacromolecules 12:3612–3620. https://doi.org/10.1021/bm200876x
Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S (2009) Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery. Biomaterials 30:5757–5766. https://doi.org/10.1016/j.biomaterials.2009.07.020
Rao N V, Mane SR, Kishore A, Sarma JD, Shunmugam R (2012) Norbornene derived doxorubicin copolymers as drug carriers with pH responsive hydrazone linker. Biomacromolecules 13:221–230. https://doi.org/10.1021/bm201478k
Fu C, Li H, Li N, Miao X, Xie M, Du W, Zhang L-M (2015) Conjugating an anticancer drug onto thiolated hyaluronic acid by acid liable hydrazone linkage for its gelation and dual stimuli-response release. Carbohydr Polym 128:163–170. https://doi.org/10.1016/j.carbpol.2015.04.024
Christie RJ, Anderson DJ, Grainger DW (2010) Comparison of hydrazone heterobifunctional crosslinking agents for reversible conjugation of thiol-containing chemistry. Bioconjug Chem 21(10):1779–1787. https://doi.org/10.1021/bc100049c
West KR, Otto S (2005) Reversible covalent chemistry in drug delivery. Curr Drug Discov Technol 2:123–160. https://doi.org/10.2174/1570163054866882
Filomeni G, Rotilio G, Ciriolo MR (2002) Cell signalling and the glutathione redox system. Biochem Pharmacol 64:1057–1064. https://doi.org/10.1016/S0006-2952(02)01176-0
Tu Y, Peng F, White PB, Wilson DA (2017) Redox-sensitive stomatocyte nanomotors: destruction and drug release in the presence of glutathione. Angew Chem Int Ed 56:7620–7624. https://doi.org/10.1002/anie.201703276
Acknowledgments
The authors gratefully acknowledge for support of the Faculty of Chemical Engineering, Industrial University of Ho Chi Minh City.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, X.T., Vu-Quang, H., Doan, VD. et al. One-step approach of dual-responsive prodrug nanogels via Diels-Alder reaction for drug delivery. Colloid Polym Sci 299, 675–683 (2021). https://doi.org/10.1007/s00396-020-04789-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-020-04789-z