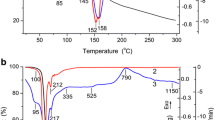
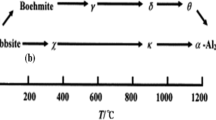
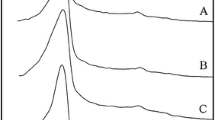
Abstract
In this paper, α-alumina structures were successfully prepared via hydrothermal synthesis supported with sodium dodecyl sulfonate anionic surfactant. The effect of the surfactant and the calcination time were investigated. The characterization of the samples calcinated at 1200 °C was performed using Raman spectroscopy, X-Ray Difraction analysis, Fourier transform infrared spectroscopy, thermogravimetric analysis, and scanning electron microscopy techniques. Experimental results showed that pure α-Al2O3 structures were obtained with different morphologies.







Similar content being viewed by others
References
Chandradass J, Yoon JH, Bae D-s (2008) Synthesis and characterization of zirconia doped alumina nanopowder by citrate–nitrate process. Mater Sci Eng A 473:360–364
Sun Z-X, Zheng T-T, Bo Q-B, Du M, Forsling W (2008) Effects of calcination temperature on the pore size and wall crystalline structure of mesoporous alumina. J Colloid Interface Sci 319:247–251
Hotta M, Kondo N, Kita H, Ohji T, Izutsu Y, Arima T, Matsumura Y (2015) Joining of alumina with an alumina–zirconia insert under low mechanical pressure. J Asian Ceramic Soc 3(1):59–63
Zhoua W, Niu X, Min G, Song Z, Zhang J, Liua Y, Li X, Zhang J, Feng S (2009) Porous alumina nano-membranes: Soft replica molding for large area UV-nanoimprint lithography. Microelectron Eng 86(12):2375–2380
Yang J-Z, Fang M-H, Huang Zo-H, Hu X-Z, Liu Y-G, Sun H-R, Huang J-T, Li X-C (2012) Solid particle impact erosion of alumina-based refractories at elevated temperatures. J Eur Ceram Soc 32(2):283–289
Kir’yanov AV, Siddiki SH, Barmenkov YO, Das S, Dutta D, Dhar A, Khakhalin AV, Sholokhov EM, Il’ichev NN, Didenko SI, Paul MC (2017) Hafnia-yttria-alumina-silica based optical fibers with diminished mid-IR (> 2 μm) loss. Opt Mater Express 7(7):2511–2518
Levin I, Brandon D (1998). J Am Ceram Soc 81:1995–2012
Ravanchi MT, Fard MR, Fadaeerayeni S, Yaripour F (2015) Effect of calcination conditions on crystalline structure and pore size distribution for a mesoporous alumina. J Chem Eng Commun 202(4):493–499
Dwivedi RK, Gowda G (1985) Thermal stability of aluminium oxides prepared from gel. J Mater Sci Lett 4:331–334
Saraswati V, Rao GVN, Rao GVR (1987) Structural evolution in alumina gel. J Mater Sci 22:2529–2534
Assih T, Ayral A, Abenoza M, Phalippou J (1988) Raman study of alumina gels. J Mater Sci 23:3326–3331
Ozao R, Ochiai M, Yoshida H, Ichimura Y, Inada T, Therm J (2001). Anal Calorim 64:923–932
Reid CB, Forrester JS, Goodshaw HJ, Kisi EH, Suaning GJ (2008) A study in the mechanical milling of alumina powder. Ceram Int 34(6):1551–1556
Forrester JS, Goodshaw HJ, Kisi EH, Suaning GJ, Zobec JS (2008). J Aust Ceram Soc 44(1):47–52
Chou TC, Nieh TG (1991) Nucleation and concurrent anomalous grain growth of alpha-Al2O3 during gamma - alpha phase transformation. J Am Ceram Soc 74(9):2270–2279
Varma HK, Mani TV, Damodran AD (1994) Characteristics of alumina powders prepared by spray-drying of boehmite sol. J Am Ceram Soc 77(6):1597–1600
Lafficher R, Digne M, Salvatori F, Boualleg M, Colson D, Puel F (2017) Development of new alumina precipitation routes for catalysis applications. J Cryst Growth 468:526–530
Mirjalili F, Hasmaliza M, Abdullah LC (2010) Size-controlled synthesis of nano α-alumina particles through the sol–gel method. Ceram Int 36:1253–1257
Panda PK, Jaleel VA, Usha Devi S (2006) Hydrothermal synthesis of boehmite and α-alumina from Bayer’s alumina trihydrate. J Mater Sci 41(24):8386–8389
Bhaduri S, Zhou E, Bhaduri SB (1996) Auto ignition processing of nanocrystalline α-Al2O3. Nanostruct Mater 7(5):487–496
Li J, Pan Y, Xiang C, Ge Q, Guo J (2006) Low temperature synthesis of ultrafine α-Al2O3 powder by a simple aqueous sol–gel process. Ceram Int 32:587–591
Vural S (2007) Formation of nanometric metal oxide sols, structural control and physicochemical characterization. Masters Dissertation, Inonu University
Lu AH, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and zpplication. Angew Chem Int Ed 46:1222–1244
Behera PS, Sarkar R, Bhattacharyya S (2016) Nano alumina: a review of the powder synthesis method. Interceram-International Ceramic Review 65(1–2):10–16
Zhu Z, Liu H, Sun H, Yang D (2009) Surfactant assisted hydrothermal and thermal decomposition synthesis of alumina microfibers with mesoporous structure. Chem Eng J 155:925–930
Ghanizadeh S, Bao X, Vaidhyanathan B, Binner J (2014) Synthesis of nano α-alumina powders using hydrothermal and precipitation routes: a comparative study. Ceram Int 40:1311–1319
Khazaei A, Nazari S, Karimi G, Ghaderi E, Moradian KM, Bagherpor Z, Nazari S (2016). Int J Nanosci Nanotechnol 12(4):207–214
Tabesh S, Davar F, Loghman-Estarki MR (2018) Preparation of γ-Al2O3 nanoparticles using modified sol-gel method and its use for the adsorption of lead and cadmium ions. J Alloys Compd 730:441–449
Aguado J, Escola JM, Castro MC, Paredes B (2005) Sol–gel synthesis of mesostructured γ-alumina templated by cationic surfactants. Microporous Mesoporous Mater 83(1–3):181–192
Cava S, Tebcherani SM, Souza IA, Pianaro SA, Paskocimas CA, Longo E, Varela JA (2007) Structural characterization of phase transition of Al2O3 nanopowders obtained by polymeric precursor method. Mater Chem Phys 103(55):394–399
Gangwar J, Gupta BK, Tripathi SK, Srivastav AK (2015) Phase dependent thermal and spectroscopic responses of Al2O3nanostructures with different morphogenesis. Nanoscale 7:13313–13344
Pezzotti G, Zhua W (2015) Resolving stress tensor components in space from polarized Raman spectra: polycrystalline alumina. Phys Chem Chem Phys 17:2608–2627
Bawa SG, Ahmed AS, Okonkwo PC (2017). Nig J Technol 36(3):822–828
Zhou SX, Antonietti M, Niederberger M (2007) Low-temperature synthesis of γ-alumina nanocrystals from aluminum acetylacetonate in nonaqueous media. Small 3:763–767
Djebaili K, Mekhalif Z, Boumaza A, Djelloul A (2015) J Spectro Article ID 868109. 16 pages. https://doi.org/10.1155/2015/868109
Sicard L, Llewellyn PL, Patarin J, Kolenda F (2001) Investigation of the mechanism of the surfactant removal from a mesoporous alumina prepared in the presence of sodium dodecylsulfate. Micro Meso Mater 44-45:195–201
Qu L, He C, Yang Y, He Y, Liu Z (2005) Hydrothermal synthesis of alumina nanotubes templated by anionic surfactant. Mater Lett 59:4034–4037
Márquez-Alvarez C, Žilková N, Pérez-Pariente J (2008) Synthesis, characterization and catalytic aqpplications of organized mesoporous aluminas. Catal Rev Sci Eng 50(2):222–286
Valange S, Guth J-L, Kolenda F, Lacombe S, Gabelica Z (2000). Microporous Mesoporous Mater 35–36:597–607
Funding
This work is financially supported by the Necmettin Erbakan University Research Fund (Project No: BAP- 181331002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vural, S., Sari, Ö. Synthesis and characterization of SDS assistant α-alumina structures and investigation of the effect of the calcination time on the morphology. Colloid Polym Sci 297, 107–114 (2019). https://doi.org/10.1007/s00396-018-4442-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4442-4