Abstract
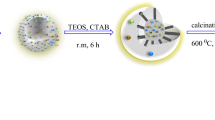
Yolk-shell nanocomposite particles (YSNPs) have been extensively investigated because of their great application potential and unique properties attributed to their distinct structures and multiple discrete functions. In this study, Fe3O4@Void@PMAA@Void@TiO2, a special YSNP with double shells and dual cavities (denoted as DDYSNPs), was prepared by conducting dispersion polymerization with a high production rate and by using a hydrothermal strategy. The synthesized DDYSNPs, which effectively integrate inorganic and organic functions, exhibit an outstanding photocatalytic activity under ultraviolet light irradiation for five experimental cycles and show an excellent adsorptive property for heavy metal ions. These comprehensive properties are mainly attributed to the novel structure of double-shelled and dual-cavity structures.

ᅟ










Similar content being viewed by others
References
Zhang F, Braun GB, Shi YF, Zhang YC, Sun XH, Reich NO, Zhao DY, Stucky G (2010) Fabrication of Ag@SiO2@Y2O3:Er nanostructures for bioimaging: tuning of the upconversion fluorescence with silver nanoparticles. J Am Chem Soc 132(9):2850. https://doi.org/10.1021/ja909108x
Zhang LY, Wang TT, Yang L, Liu C, Wang CG, Liu HY, Wang YA, Su ZM (2012) General route to multifunctional uniform yolk/mesoporous silica shell nanocapsules: a platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Chem Eur J 18(39):12512–12,521. https://doi.org/10.1002/chem.201200030
Huang PL, Zeng BZ, Mai ZX, Deng JT, Fang YP, Huang WH, Zhang HW, Yuan JY, Wei Y, Zhou WY (2016) Novel drug delivery nanosystems based on out-inside bifunctionalized mesoporous silica yolk-shell magnetic nanostars used as nanocarriers for curcumin. J Mater Chem B 4(1):46–56. https://doi.org/10.1039/c5tb02184g
Yang L, Guo GN, Sun HJ, Shen XD, Hu JH, Dong AG, Yang D (2016) Ionic liquid as the C and N sources to prepare yolk-shell Fe3O4@N-doped carbon nanoparticles and its high performance in lithium-ion battery. Electrochim Acta 190:797–803. https://doi.org/10.1016/j.electacta.2016.01.028
Jin J, Huang SZ, Li Y, Tian H, Wang HE, Yu Y, Chen LH, Hasan T, Su BL (2015) Hierarchical nanosheet-constructed yolk-shell TiO2 porous microspheres for lithium batteries with high capacity, superior rate and long cycle capability. Nanoscale 7(30):12979–12,989. https://doi.org/10.1039/c5nr02800k
Liu N, Wu H, McDowell MT, Yao Y, Wang CM, Cui Y (2012) A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett 12(6):3315–3321. https://doi.org/10.1021/nl3014814
Ham MH, Paulus GLC, Lee CY, Song C, Kalantar-zadeh K, Choi W, Han JH, Strano MS (2010) Evidence for high-efficiency exciton dissociation at polymer/single-walled carbon nanotube interfaces in planar nano-heterojunction photovoltaics. ACS Nano 4(10):6251–6259. https://doi.org/10.1021/nn1019384
Liu J, Qiao SZ, Hartono SB, Lu GQ (2010) Monodisperse yolk-shell nanoparticles with a hierarchical porous structure for delivery vehicles and nanoreactors. Angew Chem Int Ed 49(29):4981–4985. https://doi.org/10.1002/anie.201001252
Wu S, Kaiser J, Drechsler M, Ballauff M, Lu Y (2013) Thermosensitive Au-PNIPA yolk-shell particles as “nanoreactors” with tunable optical properties. Colloid Polym Sci 291(1):231–237. https://doi.org/10.1007/s00396-012-2736-5
Sun Q, Guo CZ, Wang GH, Li WC, Bongard HJ, Lu AH (2013) Fabrication of magnetic yolk–shell nanocatalysts with spatially resolved functionalities and high activity for nitrobenzene hydrogenation. Chem Eur J 19(20):6217–6220. https://doi.org/10.1002/chem.201300307
Liu WJ, Liu YX, Yan XY, Yong GP, Xu YP, Liu SM (2014) One-pot synthesis of yolk shell mesoporous carbon spheres with high magnetisation. J Mater Chem A 2(25):9600–9606. https://doi.org/10.1039/c4ta01088d
Wang Y, Li L, Wang CG, Wang TT (2015) Facile approach to synthesize uniform Au@ mesoporous SnO2 yolk-shell nanoparticles and their excellent catalytic activity in 4-nitrophenol reduction. J Nanopart Res 18(1):11. https://doi.org/10.1007/s11051-015-3307-8
Wang C, Chen JC, Zhou XR, Li W, Liu Y, Yue Q, Xue ZT, Li YH, Elzatahry AA, Deng YH, Zhao DY (2015) Magnetic yolk-shell structured anatase-based microspheres loaded with Au nanoparticles for heterogeneous catalysis. Nano Res 8(1):238–245. https://doi.org/10.1007/s12274-014-0647-0
Purbia R, Paria S (2015) Yolk/shell nanoparticles: classifications, synthesis, properties, and applications. Nanoscale 7(47):19789–19,873. https://doi.org/10.1039/c5nr04729c
Lee I, Joo JB, Yin YD, Zaera F (2011) A yolk@shell nanoarchitecture for Au/TiO2 catalysts. Angew Chem Int Ed 50(43):10208–10,211. https://doi.org/10.1002/anie.201007660
Liu HY, Wang TT, Zhang LY, Li L, Wang YA, Wang CG, Su ZM (2012) Selected-control fabrication of multifunctional fluorescent-magnetic core-shell and yolk-shell hybrid nanostructures. Chem Eur J 18(12):3745–3752. https://doi.org/10.1002/chem.201103066
Ma PC, Jiang W, Wang FH, Li FS, Shen P, Chen MD, Wang YJ, Liu J, Li PY (2013) Synthesis and photocatalytic property of Fe3O4@TiO2 core/shell nanoparticles supported by reduced graphene oxide sheets. J Alloys Compd 578:501–506. https://doi.org/10.1016/j.jallcom.2013.07.026
Song HJ, You SS, Chen T, Jia XH (2015) Controlled preparation of TiO2 hollow microspheres constructed by crosslinked nanochains with high photocatalytic activity. J Mater Sci Mater Electron 26(11):8442–8450. https://doi.org/10.1007/s10854-015-3513-2
Chen LL, Li L, Wang TT, Zhang LY, Xing SX, Wang CG, Su ZM (2014) A novel strategy to fabricate multifunctional Fe3O4@C@TiO2 yolk-shell structures as magnetically recyclable photocatalysts. Nanoscale 6(12):6603–6608. https://doi.org/10.1039/c4nr00175c
Kim EJ, Lee CS, Chang YY, Chang YS (2013) Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl Mater Interfaces 5(19):9628–9634. https://doi.org/10.1021/am402615m
Yue YF, Mayes RT, Gill G, Kuo LJ, Wood J, Binder A, Brown S, Dai S (2015) Macroporous monoliths for trace metal extraction from seawater. RSC Adv 5(62):50005–50,010. https://doi.org/10.1039/c5ra02131f
Saito T, Brown S, Chatterjee S, Kim J, Tsouris C, Mayes RT, Kuo LJ, Gill G, Oyola Y, Janke CJ, Dai S (2014) Uranium recovery from seawater: development of fiber adsorbents prepared via atom-transfer radical polymerization. J Mater Chem A 2(35):14674–14,681. https://doi.org/10.1039/c4ta03276d
Li H, Li W, Zhang Y, Wang T, Wang B, Xu W, Jiang L, Song W, Shu C, Wang C (2011) Chrysanthemum-like α-FeOOH microspheres produced by a simple green method and their outstanding ability in heavy metal ion removal. J Mater Chem 21(22):7878–7881. https://doi.org/10.1039/C1JM10979K
Ayranci E, Duman O (2009) Adsorption of some dyes onto activated carbon cloth. Sep Sci Technol 44(15):3735–3752. https://doi.org/10.1080/01496390903182891
Ayranci E, Duman O (2010) Structural effects on the interactions of benzene and naphthalene sulfonates with activated carbon cloth during adsorption from aqueous solutions. Chem Eng J 156(1):70–76. https://doi.org/10.1016/j.cej.2009.09.038
Duman O, Ayranci E (2010) Adsorptive removal of cationic surfactants from aqueous solutions onto high-area activated carbon cloth monitored by in situ UV spectroscopy. J Hazard Mater 174(1–3):359–367. https://doi.org/10.1016/j.jhazmat.2009.09.058
Duman O, Tunç S, Polat TG (2015) Adsorptive removal of triarylmethane dye (basic red 9) from aqueous solution by sepiolite as effective and low-cost adsorbent. Microporous Mesoporous Mater 210:176–184. https://doi.org/10.1016/j.micromeso.2015.02.040
Li GL, Tai CA, Neoh KG, Kang ET, Yang XL (2011) Hybrid nanorattles of metal core and stimuli-responsive polymer shell for confined catalytic reactions. Polym Chem 2(6):1368–1374. https://doi.org/10.1039/c1py00054c
Li YY, Dong MJ, Kong J, Chai ZH, Fu GQ (2013) Synthesis of Fe3O4@poly (methacrylic acid) core-shell submicrospheres via RAFT precipitation polymerization. J Colloid Interface Sci 394:199–207. https://doi.org/10.1016/j.jcis.2012.12.007
Duman O, Tunç S, Polat TG, Bozoğlan BK (2016) Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic methylene blue dye adsorption. Carbohydr Polym 147:79–88. https://doi.org/10.1016/j.carbpol.2016.03.099
Duman O, Tunç S, Bozoğlan BK, Polat TG (2016) Removal of triphenylmethane and reactive azo dyes from aqueous solution by magnetic carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite. J Alloys Compd 687:370–383. https://doi.org/10.1016/j.jallcom.2016.06.160
Liu J, Sun ZK, Deng YH, Zou Y, Li CY, Guo XH, Xiong LQ, Gao Y, Li FY, Zhao DY (2009) Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew Chem Int Ed 48(32):5875–5879. https://doi.org/10.1002/anie.200901566
Liu JW, Xu JJ, Che RC, Chen HJ, Liu MM, Liu ZW (2013) Hierarchical Fe3O4@TiO2 yolk-shell microspheres with enhanced microwave-absorption properties. Chem Eur J 19(21):6746–6752. https://doi.org/10.1002/chem.201203557
Li W, Zhao DY (2013) Extension of the stober method to construct mesoporous SiO2 and TiO2 shells for uniform multifunctional core-shell structures. Adv Mater 25(1):142–149. https://doi.org/10.1002/adma.201203547
Chen JS, Luan DY, Li CM, Boey FYC, Qiao SZ, Lou XW (2010) TiO2 and SnO2@TiO2 hollow spheres assembled from anatase TiO2 nanosheets with enhanced lithium storage properties. Chem Commun 46(43):8252–8254. https://doi.org/10.1039/c0cc02973d
Chen GX, Qiao CD, Wang Y, Yao JS (2014) Synthesis of magnetic gelatin and its adsorption property for Cr (VI). Ind Eng Chem Res 53(40):15576–15,581. https://doi.org/10.1021/ie502709u
Zhao LL, Liu HR, Wang FW, Zeng L (2014) Design of yolk-shell Fe3O4@PMAA composite microspheres for adsorption of metal ions and pH-controlled drug delivery. J Mater Chem A 2(19):7065–7074. https://doi.org/10.1039/c4ta00976b
Liu SW, Xia JQ, Yu JG (2015) Amine-functionalized titanate nanosheet-assembled yolk@shell microspheres for efficient cocatalyst-free visible-light photocatalytic CO2 reduction. ACS Appl Mater Interfaces 7(15):8166–8175. https://doi.org/10.1021/acsami.5b00982
Zheng J, Cheng C, Yan RW, Fang WJ, Chen C, Huai HX, Wang CC (2014) Synthesis of yolk-shell magnetic magnesium silicate with tunable yolk morphology for removal of methylene blue in water. J Alloys Compd 596:5–9. https://doi.org/10.1016/j.jallcom.2014.01.164
Falahatdoost S, Ara MHM, Shaban Z, Ghazyani N (2015) Optical investigation of shell thickness in light scattering SiO2 particle with TiO2 nanoshells and its application in dye sensitized solar cells. Opt Mater 47:51–55. https://doi.org/10.1016/j.optmat.2015.06.053
Yu YQ, Yan L, Cheng JM, Jing CY (2017) Mechanistic insights into TiO2 thickness in Fe3O4@TiO2-GO composites for enrofloxacin photodegradation. Chem Eng J 325:647–654. https://doi.org/10.1016/j.cej.2017.05.092
Pan JQ, Li XY, Zhao QD, Li TT, Tade M, Liu SM (2015) Construction of Mn0.5Zn0.5Fe2O4 modified TiO2 nanotube array nanocomposite electrodes and their photoelectrocatalytic performance in the degradation of 2,4-DCP. J Mater Chem C 3(23):6025–6034. https://doi.org/10.1039/c5tc01008j
Zhang MM, Li XY, Zhao QD, Fan SY, Jiang Z, Chen GH (2017) AgInS2 nanoparticles modified TiO2 nanotube array electrodes: ultrasonic-assisted SILAR preparation and mechanism of enhanced photoelectrocatalytic activity. Mol Catal 442:97–106. https://doi.org/10.1016/j.mcat.2017.09.009
Houas A, Lachheb H, Ksibi M, Elaloui W, Guilard C, Herrmann JM (2001) Photocatalytic degradation pathway of methylene blue in water. Appl Catal B Environ 31(2):145–157. https://doi.org/10.1016/S0926-3373(00)00276-9
Zhang XQ, Zhu YH, Yang XL, Wang SW, Shen JH, Lin BB, Li CZ (2013) Enhanced visible light photocatalytic activity of interlayer-isolated triplex Ag@SiO2@TiO2 core-shell nanoparticles. Nanoscale 5(8):3359–3366. https://doi.org/10.1039/c3nr00044c
Tian P, Han XY, Ning GL, Fang HX, Ye JW, Gong WT, Lin Y (2013) Synthesis of porous hierarchical MgO and its superb adsorption properties. ACS Appl Mater Interfaces 5(23):12411–12,418. https://doi.org/10.1021/am403352y
Su WK, Zhang T, Li L, Xing J, He MY, Zhong YJ, Li ZQ (2014) Synthesis of small yolk-shell Fe3O4@TiO2 nanoparticles with controllable thickness as recyclable photocatalysts. RSC Adv 4(17):8901–8906. https://doi.org/10.1039/c3ra47461e
Liu YB, Wang YQ, Zhou SM, Lou SY, Yuan L, Gao T, Wu XP, Shi XJ, Wang K (2012) Synthesis of high saturation magnetization superparamagnetic Fe3O4 hollow microspheres for swift chromium removal. ACS Appl Mater Interfaces 4(9):4913–4920. https://doi.org/10.1021/am301239u
Adegoke HI, AmooAdekola F, Fatoki OS, Ximba BJ (2014) Adsorption of Cr (VI) on synthetic hematite (α-Fe2O3) nanoparticles of different morphologies. Korean J Chem Eng 31(1):142–154. https://doi.org/10.1007/s11814-013-0204-7
Han X, Gai LG, Jiang HH, Zhao LC, Liu H, Zhang W (2013) Core-shell structured Fe3O4/PANI microspheres and their Cr (VI) ion removal properties. Synth Met 171:1–6. https://doi.org/10.1016/j.synthmet.2013.02.025
Setshedi KZ, Bhaumik M, Onyango MS, Maity A (2015) High-performance towards Cr (VI) removal using multi-active sites of polypyrrole-graphene oxide nanocomposites: Batch and column studies. Chem Eng J 262:921–931. https://doi.org/10.1016/j.cej.2014.10.034
Li HZ, Zhang L, Sun ZB, Liu Y, Yang B, Yan SQ (2015) One-step synthesis of magnetic 1,6-hexanediamine-functionalized reduced graphene oxide-zinc ferrite for fast adsorption of Cr(VI). RSC Adv 5(40):31787–31,797. https://doi.org/10.1039/c5ra00856e
Duman O, Ayranci E (2010) Attachment of benzo-crown ethers onto activated carbon cloth to enhance the removal of chromium, cobalt and nickel ions from aqueous solutions by adsorption. J Hazard Mater 176:231–238. https://doi.org/10.1016/j.jhazmat.2009.11.018
Funding
The work described in this paper was supported by the Shandong Province Natural Science Foundation (ZR2012EMM009, ZR2013EMQ005, and ZR2018MEM012), the Foundation of Key Laboratory of Pulp and Paper Science and Technology of Ministry of Education/Shandong Province of China (No. KF201602), the Scientific Research Foundation for the Returned Overseas Scholars in Jinan (20100406), the National Training Program of Innovation and Entrepreneurship for Undergraduates (201610431033), and the National Natural Science Foundations of China (31570566, 31500489, 51372140, 51303086, 51403111, 51503107, and 51172130).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Electronic supplementary material
ESM 1
(DOCX 750 kb)
Rights and permissions
About this article
Cite this article
Wang, YF., Yang, TT., Liu, WL. et al. Design of double-shelled and dual-cavity structures in Fe3O4@Void@PMAA@Void@TiO2 nanocomposite particles for comprehensive photocatalyst and adsorbent applications. Colloid Polym Sci 296, 1719–1728 (2018). https://doi.org/10.1007/s00396-018-4390-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4390-z