Abstract
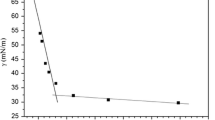
The paper provided a new nonionic-anionic silicone surfactant—comb-like polysiloxanes with oligo(oxyethylene) and sulfonate groups (ESPS) in side chains, which was synthesized by successive hydrosilylation of poly(dimethylhydro)siloxane with allyl poly(ethylene glycol) acetic ester (M n = 540) and allyl glycidyl ether, then ring-opening reaction of oxirane groups with sodium bisulfite. 1H NMR, FT-IR, and gel permeation chromatography (GPC) were used to confirm the chemical structure of the surfactant. The thermal transition behavior of ESPS studied with differential scanning calorimetry (DSC) analysis showed that the surfactant had low glass-transition temperature of − 59.3 °C. Surface tension measurement result indicated that the the surface tension at the critical micelle concentration (cmc, 69.8 mg L−1) was 29.4 mN m−1, which was much lower than those of conventional hydrocarbon surfactants, indicating the higher surface activity of ESPS. The solvent-free dimethoxysilyl-terminated polypropylene oxide waterborne emulsions were prepared using ESPS as the single emulsifier via phase-inversion emulsification technique. An obvious abrupt changing point appearing at the conductivity curves indicated that the phase inversion was completely accomplished. The emulsions prepared with about 3.0 wt% ESPS content in oil phase exhibited excellent storage stability at 50 °C for 42 days and freeze-thaw stability.






Similar content being viewed by others
References
Hill RM (1999) Silicone surfactants. Marcel Dekker, New York, pp 1–48
Kawakami Y, Murthy RS, Yamashita Y (1983) Surface active properties of silicone containing polymers. Polym Bullet 10(7-8):368–372. https://doi.org/10.1007/BF00281950
Cui X, Qiao C, Wang S, Ding Y, Hao C, Li J (2015) Synthesis, surface properties, and antibacterial activity of polysiloxane quaternary ammonium salts containing epoxy group. Colloid Polym Sci 293(7):1971–1981. https://doi.org/10.1007/s00396-015-3588-6
Hou Z, Kan C (2015) Polysiloxanes with quaternary ammonium groups for SPPO aqueous emulsions. J Surfact Deterg 18(3):517–522. https://doi.org/10.1007/s11743-014-1664-4
Liu JK, Wnek GE (1994) Reaction of silyl ketene acetal-functionalized polysiloxanes: synthesis of sulfonated polysiloxanes. Macromolecules 27(15):4080–4083. https://doi.org/10.1021/ma00093a008
Snow SA, Fenton WN, Owen MJ (1990) Synthesis and characterization of zwitterionic silicone sulfobetaine surfactants. Langmuir 6(2):385–391. https://doi.org/10.1021/la00092a017
Zeng X, Wang H, Chen Y, Wang L (2015) Synthesis and solution properties of carbohydrate-modified polysiloxane bola surfactants. J Surfact Deterg 18(6):1089–1094. https://doi.org/10.1007/s11743-015-1730-6
Wang L, Zhang D, Du Z, Wang G, Wang S, Cao Y (2011) Synthesis and properties of lactobionamide-based polysiloxane surfactant. Tenside Surf Det 48(4):281–285. https://doi.org/10.3139/113.110131
Zhou Y, Zheng C, Zhan Y, Yin D (2012) The performance of polyether modified polysiloxane. Adv Mater Res 554-556:140–146. https://doi.org/10.4028/www.scientific.net/AMR.554-556.140
Zhao J, An Q, Li X, Huang L, Xu X (2015) A comblike polysiloxane with pendant quaternary ammonium polyether groups: its synthesis, physical properties and antibacterial performance. J Polym Res 22(9):174–182. https://doi.org/10.1007/s10965-015-0821-4
Hou Z, Yang B, Zhang D, Xu Z, Kan C (2016) Polysiloxanes with quaternary ammonium and polyether groups for silyl-terminated polypropylene oxide waterborne emulsions. J Surfact Deterg 19(4):739–745. https://doi.org/10.1007/s11743-016-1825-8
Kuo PL, Hou SS, Teng CK, Liang WJ (2001) Function and performance of silicone copolymer (VI): synthesis and novel solution behavior of water-soluble polysiloxanes with different hydrophiles. Colloid Polym Sci 279(3):286–291. https://doi.org/10.1007/s003960000448
Kojima S, Watanabe Y (1993) Development of high performance, waterborne coatings. Part I: emulsification of epoxy resin. Polym Eng Sci 33(5):253–259. https://doi.org/10.1002/pen.760330502
Bayrak Y, Iscan M (2004) Phase inversion temperatures of triton X-100/1-butanol /hydrocarbon/water systems. J Surfact Deterg 7(4):363–366. https://doi.org/10.1007/s11743-004-0319-5
Chen H, Zhu B (2014) A new anionic oxalamide lauryl succinate sodium sulfonate gemini surfactant: microwave-assisted synthesis and surface activities. J Surfact Deterg 17(5):937–942. https://doi.org/10.1007/s11743-014-1585-2
Zhu J, Gosen C, Marchant R (2006) Synthesis and characterization of poly(vinyl amine)-based amphiphilic comb-like dextran glycopolymers by a two-step method. J Polym Sci A Polym Chem 44(1):192–199. https://doi.org/10.1002/pola.20998
Ryu HS, Kim DG, Lee JC (2010) Polysiloxanes containing polyhedral oligomeric silsesquioxane groups in the side chains; synthesis and properties. Macromol Res 18(10):1021–1029. https://doi.org/10.1007/s13233-010-1006-y
Lee JH, Go AK, SH O, Lee KE, Yuk SH (2005) Tissue anti-adhesion potential of ibuprofen-loaded PLLA-PEG diblock copolymer films. Biomaterials 26(6):671–678. https://doi.org/10.1016/j.biomaterials.2004.03.009
Subramani S, Lee JM, Lee JY (2007) Synthesis and properties of room temperature curable trimethoxysilane-terminated polyurethane and their dispersions. Polym Adv Technol 18(8):601–609. https://doi.org/10.1002/pat.860
Hou Z, Qu W, Kan C (2015) Synthesis and properties of triethoxysilane-terminated anionic polyurethane and its waterborne dispersions. J Polym Res 22(6):111–119. https://doi.org/10.1007/s10965-015-0757-8
Yang Z, Xu Y, Zhao D, Xu M (2000) Preparation of waterborne dispersions of epoxy resin by the phase-inversion emulsification technique. I. Experimental study on the phase-inversion process. Colloid Polym Sci 278(12):1164–1171. https://doi.org/10.1007/s003960000375
Funding
This study was funded by the National Natural Science Funds of China (grant number 21606138).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, J., Teng, H., Hou, Z. et al. Comb-like polysiloxanes with oligo(oxyethylene) and sulfonate groups in side chains for solvent-free dimethoxysilyl-terminated polypropylene oxide waterborne emulsions. Colloid Polym Sci 296, 157–163 (2018). https://doi.org/10.1007/s00396-017-4237-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4237-z