Abstract
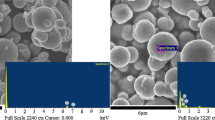
Soft magnetic carbonyl iron (CI) for application to magnetic stimuli-responsive smart materials in an external magnetic field normally implies severe sedimentation problems because of the density imbalance between the CI particles and the dispersed medium. As a new method of ameliorating this problem, CI/polydopamine (PDA) composite particles with core–shell structure were synthesized via an oxidative self-polymerization process, bringing into an account of the coating efficiency of the PDA. Surface morphology of the CI/PDA composite particles was characterized by scanning electron microscopy, while Fourier transform infrared spectroscopy, X-ray energy-dispersive spectroscopy, and vibrating sample magnetometry were adopted to measure the chemical composition, weight and atomic percentages, and magnetic properties of the fabricated composites. The magnetic stimuli-response of their magnetorheological (MR) properties was examined by using a rotational rheometer at various magnetic field strengths and compared with pristine CI-particle-based MR fluid. The measured dynamic yield stress was fitted to a universal yield stress equation well. The sedimentation properties of the CI/PDA-composite-based MR fluid were further examined by a Turbiscan™. In addition, the anti-corrosion characteristic of these particles was also investigated.













Similar content being viewed by others
References
Andrei OE, Bica I (2009) Some mechanisms concerning the electrical conductivity of magnetorheological suspensions in magnetic field. J Ind Eng Chem 15:573–577
Margida AJ, Weiss KD, Carlson JD (1996) Magnetorheological materials based on iron alloy particles. Int J Mod Phys B 10:3335–3341
de Vicente J, Klingenberg DJ, Hidalgo-Alvarez R (2011) Magnetorheological fluids: a review. Soft Matter 7:3701–3710
Pacull J, Goncalves S, Delgado AV, Duran JDG, Jimenez ML (2009) Effect of polar interactions on the magnetorheology of silica-coated magnetite suspensions in oil media. J Colloid Interf Sci 337:254–259
Bica I (2002) Damper with magnetorheological suspension. J Magn Magn Mater 241:196–200
Dodbiba G, Park HS, Okaya K, Fujita T (2008) Investigating magnetorheological properties of a mixture of two types of carbonyl iron powders suspended in an ionic liquid. J Magn Magn Mater 320:1322–1327
Bossis G, Khuzir P, Lacis S, Volkova O (2003) Yield behavior of magnetorheological suspensions. J Magn Magn Mater 258:456–458
Rankin PJ, Ginder JM, Klingenberg DJ (1998) Electro- and magneto-rheology. Curr Opin Colloid Interf 3:373–381
Bica I, Anitas EM, Averis LME, Bunoiu M (2015) Magnetodielectric effects in composite materials based on paraffin, carbonyl iron and graphene. J Ind Eng Chem 21:1323–1327
Sherman SG, Wereley NM (2013) Effect of particle size distribution on chain structures in magnetorheological fluids. IEEE Trans Magn 49:3430–3433
Felicia LJ, Philip J (2013) Probing of field-induced structures and tunable rheological properties of surfactant capped magnetically polarizable nanofluids. Langmuir 29:110–120
An HN, Groenewold J, Picken SJ, Mendes E (2014) Conformational changes of a single magnetic particle string within gels. Soft Matter 10:997–1005
Hong CH, Liu YD, Choi HJ (2013) Carbonyl iron suspension with halloysite additive and its magnetorheology. Appl Clay Sci 80–81:366–371
Hu W, Cook E, Wereley NM (2007) Energy absorber using a magnetorheological bypass valve filled with ferromagnetic beads. IEEE Trans Magn 43:2695–2697
Bica I (2009) Influence of magnetic field upon the electric capacity of a flat capacitor having magnetorheological elastomer as a dielectric. J Ind Eng Chem 15:605–609
Yang TH, Koo JH, Kim SY, Kyung KU, Kwon DS (2012) Application of magnetorheological fluids for a miniature haptic button: Experimental evaluation. J Intel Mater Syst Str 23:1025–1031
de Vicente J, Lopez-Lopez MT, Gonzalez-Caballero F, Duran JDG (2003) Rheological study of the stabilization of magnetizable colloidal suspensions by addition of silica nanoparticles. J Rheol 47:1093–1109
Wu WP, Zhao BY, Wu Q, Chen LS, Hu KA (2006) The strengthening effect of guar gum on the yield stress of magnetorheological fluid. Smart Mater Struct 15:N94–N98
Ngatu GT, Wereley NM (2007) Viscometric and sedimentation characterization of bidisperse magnetorheological fluids. IEEE Trans Magn 43:2474–2476
Fang FF, Jang IB, Choi HJ (2007) Single-walled carbon nanotube added carbonyl iron suspension and its magnetorheology. Diam Relat Mater 16:1167–1169
Hu B, Fuchs A, Huseyin S, Gordaninejad F, Evrensel C (2006) Atom transfer radical polymerized MR fluids. Polymer 47:7653–7663
Cvek M, Mrlik M, Ilcikova M, Plachy T, Sedlacik M, Mosnacek J, Pavlinek V (2015) A facile controllable coating of carbonyl iron particles with poly(glycidyl methacrylate): a tool for adjusting MR response and stability properties. J Mater Chem C 3:4646–4656
Machovsky M, Mrlik M, Kuritka I, Pavlinek V, Babayan V (2014) Novel synthesis of core-shell urchin-like ZnO coated carbonyl iron microparticles and their magnetorheological activity. RSC Adv 4:996–1003
Bombard AJF, Knobel M, Alcantara MR (2007) Phosphate coating on the surface of carbonyl iron powder and its effect in magnetorheological suspensions. Int J Mod Phys B 21:4858–4867
Pu H, Jiang F, Wang Y, Yan B (2010) Soft magnetic composite particles of reduced iron coated with poly(p-xylylene) via chemical vapor deposition polymerization. Colloids Surf A 361:62–65
Mrlik M, Ilcikova M, Sedlacik M, Mosnacek J, Peer P, Filip P (2014) Cholesteryl-coated carbonyl iron particles with improved anti-corrosion stability and their viscoelastic behavior under magnetic field. Colloid Polym Sci 292:2137–2143
Liu YL, Ai KL, Lu LH (2014) Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev 114:5057–5115
Dalsin JL, Hu BH, Lee BP, Messersmith PB (2003) Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc 125:4253–4258
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430
Statz AR, Meagher RJ, Barron AE, Messersmith PB (2005) New peptidomimetic polymers for antifouling surfaces. J Am Chem Soc 127:7972–7973
An P, Zuo F, Li XH, Wu YP, Zhang JH, Zheng ZH, Ding XB, Peng YX (2013) A bio-inspired polydopamine approach to preparation of gold-coated Fe3O4 core-shell nanoparticles: synthesis, characterization and mechanism. Nano 8:1350061
Zhang YW, Zhang Y, Wang H, Shen GL, Yu RQ (2009) Fabrication of a novel enzyme immobilization platform using bio-mimetic dopamine polymer films and nanogold particles. Acta Chim Sinica 67:2375–2380
Sureshkumar M, Siswanto DY, Lee CK (2010) Magnetic antimicrobial nanocomposite based on bacterial cellulose and silver nanoparticles. J Mater Chem 20:6948–6955
Fei B, Qian BT, Yang ZY, Wang RH, Liu WC, Mak CL, Xin JH (2008) Coating carbon nanotubes by spontaneous oxidative polymerization of dopamine. Carbon 46:1795–1797
Wang WC, Jiang Y, Wen SP, Liu L, Zhang LQ (2012) Preparation and characterization of polystyrene/Ag core-shell microspheres—a bio-inspired poly(dopamine) approach. J Colloid Interf Sci 368:241–249
Peng HP, Liang RP, Zhang L, Qiu JD (2013) Facile preparation of novel core-shell enzyme-Au-polydopamine-Fe3O4 magnetic bionanoparticles for glucose sensor. Biosens Bioelectron 42:293–299
Yang FC, Dong Y, Guo ZG (2014) Facile fabrication of core shell Fe3O4@polydopamine microspheres with unique features of magnetic control behavior and special wettability. Colloid Surf A 463:101–109
Liu R, Guo YL, Odusote G, Qu FL, Priestley RD (2013) Core-shell Fe3O4 polydopamine nanoparticles serve multipurpose as drug carrier, catalyst support and carbon adsorbent. ACS Appl Mater Interfaces 5:9167–9171
Yu F, Chen SG, Chen Y, Li HM, Yang L, Chen YY, Yin YS (2010) Experimental and theoretical analysis of polymerization reaction process on the polydopamine membranes and its corrosion protection properties for 304 Stainless Steel. J Mol Struct 982:152–161
Wilker JJ (2010) Marine bioinorganic materials: mussels pumping iron. Curr Opin Chem Biol 14:276–283
de Vicente J, Lopez-Lopez MT, Duran JDG, Gonzalez-Caballero F (2004) Shear flow behavior of confined magnetorheological fluids at low magnetic field strengths. Rheol Acta 44:94–103
Tian Y, Jiang JL, Meng YG, Wen SZ (2010) A shear thickening phenomenon in magnetic field controlled-dipolar suspensions. Appl Phys Lett 97:151904
Mrlik M, Ilcikova M, Pavlinek V, Mosnacek J, Peer P, Filip P (2013) Improved thermooxidation and sedimentation stability of covalently-coated carbonyl iron particles with cholesteryl groups and their influence on magnetorheology. J Colloid Interf Sci 396:146–151
Park BJ, Fang FF, Zhang K, Choi HJ (2010) Polymer-coated magnetic carbonyl iron microparticles and their magnetorheological characteristics. Korean J Chem Eng 27:716–722
Wereley NM, Chaudhuri A, Yoo JH, John S, Kotha S, Suggs A, Radhakrishnan R, Love BJ, Sudarshan TS (2006) Bidisperse magnetorheological fluids using Fe particles at nanometer and micron scale. J Intel Mat Syst Str 17:393–401
Choi HJ, Cho MS, Kim JW, Kim CA, Jhon MS (2001) A yield stress scaling function for electrorheological fluids. Appl Phys Lett 78:3806–3808
Ginder JM, Davis LC, Elie LD (1996) Rheology of magnetorheological fluids: models and measurements. Int J Mod Phys B 10:3293–3303
Hato MJ, Choi HJ, Sim HH, Park BO, Ray SS (2011) Magnetic carbonyl iron suspension with organoclay additive and its magnetorheological properties. Colloid Surf A 377:103–109
Cho MS, Lim ST, Jang IB, Choi HJ, Jhon MS (2004) Encapsulation of spherical iron-particle with PMMA and its magnetorheological particles. IEEE Trans Magn 40:3036–3038
Fang FF, Choi HJ, Seo Y (2010) Sequential coating of magnetic carbonyl iron particles with polystyrene and multiwalled carbon nanotubes and its effect on their magnetorheology. ACS Appl Mater Interfaces 2:54–60
Gandhi F, Bullough WA (2005) On the phenomenological modeling of electrorheological and magnetorheological fluid preyield behavior. J Intel Mat Syst Str 16:237–248
Park BJ, Kim TH, Choi HJ, Lee JH (2007) Emulsion polymerized polystyrene/montmorillonite nanocomposite and its viscoelastic characteristics. J Macromol Sci B 46:341–354
Schwarzl FL (1975) Numerical calculation of stress relaxation modulus from dynamic data for linear viscoelastic materials. Rheol Acta 14:581–590
Zhang WL, Choi HJ (2012) Silica-graphene oxide hybride composite particles and their electroresponsive characteristics. Langmuir 28:7055–7062
Fang FF, Liu YD, Choi HJ, Seo YS (2011) Core-shell structured carbonyl iron microspheres prepared via dual-step functionality coatings and their magnetorheological response. ACS Appl Mater Interfaces 3:3487–3495
Kim SY, Kwon SH, Liu YD, Lee JS, You CY, Choi HJ (2014) Core–shell-structured cross-linked poly(glycidyl methacrylate)-coated carbonyl iron microspheres and their magnetorheology. J Mater Sci 49:1345–1352
Acknowledgments
This research was supported by Ministry of Trade, Industry & Energy, Republic of Korea (# 10047791).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, Y.H., Ahn, W.J., Choi, H.J. et al. Fabrication and magnetic stimuli-response of polydopamine-coated core–shell structured carbonyl iron microspheres. Colloid Polym Sci 294, 329–337 (2016). https://doi.org/10.1007/s00396-015-3786-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3786-2