Abstract
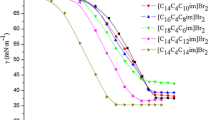
Structural versatility is an important reason for the interest in ionic liquids (ILs) and ionic-liquid-based surfactants, ILBSs. We report here on the synthesis, characterization, and micellar properties of a series of ILBSs that carry unsaturation in the head group, 1-Cn-3-vinlyimidazolium bromide, CnVnImBr, Cn = C10, C12, C14, and C16, respectively. We studied this series at 298.15 K using surface tension, ultraviolet–visible (UV–vis) spectroscopy, and steady state fluorescence of solubilized methyl orange, MO, and pyrene, respectively. We studied the electrical conductance of CnVnImBr at 298.15 to 313.15 K. From the results of surface tension and conductivity, we calculated the area per surfactant at solution/air interface; the critical micelle concentration (cmc); the degree of counter-ion binding; and the enthalpy, entropy, and free energy of micellization. These properties showed the expected dependence on the length of Cn, and indicated that micellization is an entropy-driven process. We used fluorescence data to calculate the cmc, microscopic polarity of the interfacial region, and the micelle aggregation number. The UV–vis spectra of MO were used to calculate the cmc and probe dye–ILBS interactions in the pre- and post-micellar regimes. The aggregation behavior of C16VnImBr was compared with its saturated counterpart 1-(n-hexadecyl)-3-ethylimidazolium bromide, with 1-Cn-3-methylimidazolium bromides, and with “conventional” cationic surfactants, alkyltrimethylammonium bromides. The vinyl group is less hydrophobic than the ethyl moiety.






Similar content being viewed by others
Abbreviations
- A min :
-
Area per surfactant molecule at air–water interface
- db:
-
Double bond
- cmc:
-
Critical micelle concentration
- CPC:
-
1-Cetylpyridinium chloride
- CnVnImBr:
-
1-(n-Alkyl)-3-vinylimidazolium bromide
- C16EtImBr:
-
1-(n-Hexadecyl)-3-ethylimidazolium bromide
- CnMeImBr:
-
1-(n-Alkyl)-3-methylimidazolium bromide
- CnMe3ABr:
-
N-(n-Alkyl)-N,N,N-trimethylammonium bromide
- ΔG 0m :
-
Standard free energy of micelle formation
- ΔH 0m :
-
Standard enthalpy of micelle formation
- ILs:
-
Ionic liquids
- ILBS:
-
Ionic-liquid-based surfactant
- MO:
-
Methyl orange
- N agg :
-
Micelle average aggregation number
- pC 20 :
-
Surface adsorption efficiency
- ΔS 0m :
-
Standard entropy of micelle formation
- β:
-
Fraction of micelle-bound counter-ion
- γ:
-
Surface tension
- γcmc :
-
Surface tension at cmc
- Γmax :
-
Maximum surface excess concentration
- πcmc :
-
Surface pressure at cmc
References
Roger RD, Seddon KR (2003) Ionic Liquids as Green Solvents: Progress and Prospects, American Chemical Society, Washington, D.C.
Wasserscheid P, Welton T (2003) Ionic liquids in syntheses. VCH-Wiley, New York
Jungnickel C, Luczak J, Ranke J, Fernandez JF, Muller A, Thoming J (2008) Micelle formation of imidazolium ionic liquids in aqueous solution. Colloids Surf A Physicochem Eng Asp 316:278–284. doi:10.1016/j.colsurfa. 2007.09.020
Vanyur R, Biczok L, Miskolczy Z (2007) Micelle formation of 1-alkyl-3-methylimidazolium bromide ionic liquids in aqueous solution. Colliods Surf A Physicochem Eng Asp 299:256–261
Luczak J, Jungnickel C, Joskowska M, Thoming J, Hupka J (2009) Thermodynamics of micellization of imidazolium ionic liquids in aqueous solutions. J Colloid Interface Sci 336:111–116
Zhang H, Li K, Liang H, Wang J (2008) Spectroscopic studies of the aggregation of imidazolium-based ionic liquids. Colloids Surf A Physicochem Eng Asp 329:75–81
Cornellas A, Perez L, Comelles F, Ribosa I, Manresa A, Garcia MT (2010) Self-aggregation and antimicrobial activity of imidazolium and pyridinium based ionic liquids in aqueous solution. J Colloid Interface Sci 355:164–171
Dong B, Zhao X, Zheng LQ, Zhang J, Li N, Inoue T (2008) Aggregation behavior of long-chain imidazolium ionic liquids in aqueous solution: micellization and characterization of micelle microenvironment. Colloids Surf A Physicochem Eng Asp 317:666–672
Geng F, Liu J, Zheng L, Yu L, Li Z, Li G, Tung C (2010) Micelle formation of long-chain imidazolium ionic liquids in aqueous solution measured by isothermal titration microcalorimetry. J Chem Eng Data 55:147–151
El Seoud OA, Pires PAR, Abdel-Moghny T, Bastos EL (2007) Synthesis and micellar properties of surface-active ionic liquids: 1-alkyl-3-methylimidazolium chlorides. J Colloid Interface Sci 313:296–304
Inoue T, Ebina H, Dong B, Zheng L (2007) Electrical conductivity study on micelle formation of long-chain imidazolium ionic liquids in aqueous solution. J Colloid Interface Sci 314:236–241
Sastry NV, Vaghela NM, Aswal VK (2012) Effect of alkyl chain length and head group on surface active and aggregation behavior of ionic liquids in water. Fluid Phase Equilib 327:22–29
Wang H, Wang J, Zhang S, Xuan X (2008) Structural effects of anions and cations on the aggregation behavior of ionic liquids in aqueous solutions. J Phys Chem B 112:16682–16689
Blesic M, Lopes A, Melo E, Petrovski Z, Plechkova NV, Canongia Lopes JN, Seddon KR, Rebelo LPN (2008) On the self-aggregation and fluorescence quenching aptitude of surfactant ionic liquids. J Phys Chem B 112:8645–8650
Singh T, Kumar A (2007) Aggregation Behavior of Ionic Liquids in Aqueous Solutions: Effect of Alkyl Chain Length, Cations and Anions. J Phys Chem B 111:7843–7851
Vaghela NM, Sastry NV, Aswal VK (2011) Surface active and aggregation behavior of methylimidazolium-based ionic liquids of type [Cnmim] [X], n=4, 6, 8 and [X]=Cl−, Br−, and I− in water. Colloid Polym Sci 289:309–322
Dong B, Li N, Zheng L, Yu L, Inoue T (2007) Surface adsorption and micelle formation of surface active ionic liquids in aqueous solution. Langmuir 23:4178–4182
Ao M, Kim D (2013) Aggregation behavior of aqueous solutions of 1-Dodecyl-3-methylimidazolium salts with different halide anions. J Chem Eng Data 58:1529–1534
Anouti M, Jones J, Boisset A, Jacquemin J, Caravanier MC, Lemordant D (2009) Aggregation behavior in water of new imidazolium and pyrrolidinium alkycarboxylates protic ionic liquids. J Colloid Interface Sci 340:104–111
Rather MA, Rather GM, Pandit SA, Bhat SA, Bhat MA (2015) Determination of cmc of imidazolium based surface active ionic liquids through probe-less UV–vis spectrophotometry. Talanta 131:55–58
Cheng N, Ma X, Sheng X, Wang T, Wang R, Jiao J, Yu L (2014) Aggregation behavior of anionic surface active ionic liquids with double hydrocarbon chains in aqueous solution: Experimental and theoretical investigations. Colloids Surf A Physicochem Eng Asp 453:53–61
Modaressi A, Sifaoui H, Mielcarz M, Domanska U, Rogalski M (2007) Influence of the molecular structure on the aggregation of imidazolium ionic liquids in aqueous solutions. Colloids Surf A Physicochem Eng Asp 302:181–185
Wang X, Liu J, Yu L, Jiao J, Wang R, Sun L (2013) Surface adsorption and micelle formation of imidazolium-based zwitterionic surface active ionic liquids in aqueous solutions. J Colloid Interface Sci 391:103–110
Singh T, Rao KS, Kumar A (2012) Effect of ethylene glycol and its derivatives on the aggregation behavior of an ionic liquid 1-butyl-3-methylimidazolium octyl sulfate in aqueous medium. J Phys Chem B 116:1612–1622
Blesic M, Marques MH, Plechkova NV, Seddon KR, Rebelo LPN, Lopes A (2007) Self-aggregation of ionic liquids: micelle formation in aqueous solution. Green Chem 9:481–490
Choi YS, Shim YN, Lee J, Yoon JH, Hong CS, Cheong M, Kim HS, Jang HG, Lee JS (2011) Ionic liquids as benign catalysts for the carbonylation of amines to formamides. Appl Catal A Gen 404:87–92
Wang A, Zheng X, Zhao Z, Li C, Cui Y, Zheng X, Yin J, Yang G (2014) Bronsted acid ionic liquids catalyzed Friedel–Crafts Alkylations of electron-rich arenes with aldehydes. Appl Catal A Gen 482:198–204
Bussamara R, Melo WWM, Scholten JD, Migowski P, Marin G, Zapata MJM, Machado G, Teixeira SR, Novak MA, Dupont J (2013) Controlled synthesis of Mn3O4 nanoparticles in ionic liquids. Dalton Trans 42:14473–14479
Kim KS, Demberelnyamba D, Lee H (2004) Size-selective synthesis of gold and platinum nanoparticles using novel thiol-functionalized ionic liquids. Langmuir 20:556–560
Schutte K, Meyer H, Gemel C, Barthel J, Fischer RA, Janiak C (2014) Synthesis of Cu, Zn and Cu/Zn brass alloy nanoparticles from metal amidinate precursors in ionic liquids or propylene carbonate with relevance to methanol synthesis. Nanoscale 6:3116–3126
Yang X, Zhang S, Yu W, Liu Z, Lei L, Li N, Zhang H, Yu Y (2014) Ionic liquid-anionic surfactant based aqueous two-phase extraction for determination of antibiotics in honey by high-performance liquid chromatography. Talanta 124:1–6
Khan AB, Ali M, Malik NA, Ali A, Patel R (2013) Role of 1-methyl-3-octyl imidazolium chloride in the micellization behavior of amphiphilic drug amitriptyline hydrochloride. Colloids Surf B Biointerfaces 112:460–465
Ohno H (2011) Electrochemical aspects of ionic liquids. John Wiley & Sons Inc, New Jersey
Jana S, Parthiban A, Chai CLL (2010) Transparent, flexible and highly conductive ion gels from ionic liquid compatible cyclic carbonate network. Chem Commun 46:1488–1490
Zhao Y, Yue X, Wang X, Huang D, Chen X (2012) Micelle formation by N-alkyl-N-methylpiperidinium bromide ionic liquids in aqueous solution. Colloids Surf A Physicochem Eng Asp 412:90–95
Wei Y, Wang F, Zhang Z, Ren C, Lin Y (2014) Micellization and thermodynamic study of 1-Alkyl-3-methylimidazolium tetrafluoroborate ionic liquids in aqueous solution. J Chem Eng Data 59:1120–1129
Marrucho IM, Branco LC, Rebelo LPN (2014) Ionic liquids in pharmaceutical applications. Annu Rev Chem Biomol Eng 5:527–546
Geng F, Zheng L, Yu L, Li G, Tung C (2010) Interaction of bovine serum albumin and long-chain imidazolium ionic liquid measured by fluorescence spectra and surface tension. Process Biochem 45:306–311
Hua Y, Junyong W, Guoliang D, Aiguo Z, Hao C, Jianguo Y, Denan H (2012) Interaction mechanisms of ionic liquids [Cnmim]Br (n=4, 6, 8, 10) with bovine serum albumin. J Lumin 132:622–628
Mahajan S, Sharma R, Mahajan RK (2012) An investigation of drug binding ability of a surface active ionic liquid: micellization, electrochemical, and spectroscopic studies. Langmuir 28:17238–17246
Rao KS, Singh T, Trivedi TJ, Kumar A (2011) Aggregation behavior of amino acid ionic liquid surfactants in aqueous media. J Phys Chem 115:13847–13853
Pal A, Pillania A (2014) Self-aggregation of ionic liquid 1-butyl-2,3-imethylimidazolium tetrafluoroborate [C4mmim][BF4] in aqueous media: a conductometric, volumetric and spectroscopic study. Thermochim Acta 597:41–47
Wang X, Yu L, Jiao J, Zhang H, Wang R, Chen H (2012) Aggregation behavior of COOH-functionalized imidazolium-based surface active ionic liquids in aqueous solution. J Mol Liq 173:103–107
Kamboj R, Bharmoria P, Chauhan V, Singh S, Kumar A, Mithu VS, Kang TS (2014) Micellization behavior of morpholinium-based amide-functionalized ionic liquids in aqueous media. Langmuir 30:9920–9930
Dong B, Gao Y, Su Y, Zheng L, Xu J, Inoue T (2010) Self- aggregation behavior of fluorescent carbazole-tailed imidazolium ionic liquids in aqueous solutions. J Phys Chem B 114:340–348
Shi L, Li N, Yan H, Gao Y, Zheng L (2011) Aggregation behavior of long-chain n-aryl imidazolium bromide in aqueous solution. Langmuir 27:1618–1625
Garcia MT, Ribosa I, Perez L, Manresa A, Comelles F (2013) Aggregation behavior and antimicrobial activity of ester-functionalized imidazolium and pyridinium-based ionic liquids in aqueous solution. Langmuir 29:2536–2545
Bordes R, Tropsch J, Holmberg K (2010) Role of an amide bond for self-assembly of surfactants. Langmuir 26:3077–3083
Zhang ZQ, Xu FG, Tai S, Liu X, Mo S, Niu F (2012) Surface tension and aggregation properties of novel cationic gemini surfactants with diethyl ammonium head groups and a diamido spacer. Langmuir 28:11979–11987
Hoque J, Kumar P, Aswal VK, Haldar V (2012) Aggregation properties of amide bearing cleavable gemini surfactants by small angle neutron scattering and conductivity studies. J Phys Chem B 116:9718–9726
Hansen GE, Dennison MD (1952) The potential constants of ethane. J Chem Phys 20:313–326
Allen HC Jr, Plyler EK (1958) The structure of ethylene from infrared spectra. J Am Chem Soc 80:2673–2676
Mannhold R, Rekker RF, Dross K, Bijlo G, De Vries G (1998) The lipophilic behaviour of organic compounds. 1. An updating of the hydrophobic fragmental constant approach. Quant Struct Act Relat 17:517–536
Klevens HB (1953) Structure and aggregation in dilate solution of surface active agents. J Am Oil Chem Soc 30:74–80
Durairaj B, Blum FD (1985) Micelle formation and terminal double bonds in sodium carboxylates. J Colloid Interface Sci 106:561–564
Sprague ED, Duecker DC, Larrabee CE (1983) The effect of a terminal double bond on the micellization of a simple ionic surfactant. J Colloid Interface Sci 92:416–421
Larrabee CE, Sprague ED (1986) Aggregation of sodium undecanoate and sodium 10-undecenoate in water at 37°C: Vapor pressure osmometry. J Colloid Interface Sci 114:256–260
Damas C, Vannier L, Arabri M, Duchene A, Coudert R (1998) Micellar properties of a new series of stereochemical sodium carboxylates bearing double bonds near their ionic heads in aqueous media. J Colloid Interface Sci 198:323–329
Damas C, Vannier L, Naejus R, Coudert R (1999) Influence of structural modifications near the polar head of sodium carboxylates on their aqueous solution behavior. Colloids Surf A Physicochem Eng Asp 152:183–187
Yokoyama S, Nakagaki M (1993) Effect of double bond on the surface properties of aqueous solutions of eicosapolyenoic acids. Colloid Polym Sci 271:512–518
Kuiper JM, Buwalda RT, Hulst R, Engberts JBFN (2001) Novel pyridinium surfactants with unsaturated alkyl chains: aggregation behavior and interactions with methyl orange in aqueous solution. Langmuir 17:5216–5224
Lucero DG, Xometl OO, Palou RM, Likhanova NV, Aguilar MAD, Febles VG (2011) Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind Eng Chem Res 50:7129–7140
Luo SC, Sun S, Deorukhkar AR, Lu JT, Bhattacharyya A, Lin IJB (2011) Ionic liquids and ionic liquid crystals of vinyl functionalized imidazolium salts. J Mater Chem 21:1866–1873
Crescenzo AD, Demurtas D, Renzetti A, Siani G, Maria PD, Meneghetti M, Prato M, Fontana A (2009) Disaggregation of single-walled carbon nanotubes (SWNTs) promoted by the ionic liquid-based surfactant 1-hexadecyl-3-vinyl-imidazolium bromide in aqueous solution. Soft Matter 5:62–66
Crescenzo AD, Aschi M, Canto ED, Giordani S, Demurtas D, Fontana A (2011) Structural modifications of ionic liquid surfactants for improving the water dispersibility of carbon nanotubes: an experimental and theoretical study. Phys Chem Chem Phys 13:11373–11383
Damas C, Brembilla A, Baros F, Viriot ML, Lochon P (1994) Synthesis and behaviour study of amphiphilic polyvinylimidazolium salts in aqueous media: Effects of the microdomains on a bimolecular reaction involving hydrophobic reactants. Eur Polym J 30:1215–1222
Damas C, Baggio S, Brembilla A, Lochon P (1997) Microstructure study of new amphiphilic copolymers from 3-alkyl-1-vinylimidazolium salts. Eur Polym J 33:1219–1224
Harkins WD, Jordan HF (1930) A method for the determination of surface and interfacial tension from the maximum pull on a ring. J Am Chem Soc 52:1751–1772
Rosen MJ (2004) Surfactants and Interfacial Phenomena, 3rd edn. John Wiley & Sons, New Jersey
Zana R (1980) Ionization of cationic micelles: effect of the detergent structure. J Colloid Interface Sci 78:330–337
McAuliffe C (1966) Solubility in water of paraffin, cycloparaffin, olefin, acetylene, cycloolefin, and aromatic hydrocarbons. J Phys Chem 70:1267–1275
Tanford C (1980) The hydrophobic effect: formation of micelles and biological membranes. John Wiley, New York
Ray GB, Chakraborty I, Ghosh S, Moulik SP, Palepu R (2005) Self-aggregation of alkyltrimethylammonium bromides (C10-, C12-, C14-, and C16TAB) and their binary mixtures in aqueous medium: a critical and comprehensive assessment of interfacial behavior and bulk properties with reference to two types of micelle formation. Langmuir 21:10958–10967
Mukerjee P (1967) Nature of the association equilibriums and hydrophobic bonding in aqueous solutions of association colloids. Adv Colloid Interf Sci 1:242–275
Mukerjee P, Mysels, KJ (1972) Critical Micelle Concentrations of Aqueous Surfactant Systems. National Bureau of Standards of NSRDS-NBS 36, Washington, DC 20234.
Mosquera V, Rio JM, Attwood D, Garcia M, Jones MN, Prieto G, Suarez MJ, Sarmiento F (1998) A Study of the aggregation behavior of hexyltrimethylammonium bromide in aqueous solution. J Colloid Interface Sci 206:66–76
Bashford MT, Woolley EM (1985) Enthalpies of dilution of aqueous decyl-, dodecyl-, tetradecyl-, and hexadecyltrimethylammonium bromides at 10, 25, 40, and 55 °C. J Phys Chem 89:3173–3179
Stauff J (1983) Z Phys Chem A 183:55
Klevens HB (1952) Solubilization in alcohol—soap micelles II electrolytes as additives. J Am Chem Soc 74:4624–4626
Hafiane A, Dhahbi M, Chasseray X, Lemordant DJ (1998) J Colloid Interface Sci 205:21–25
Zhao Y, Gao S, Wang J, Tang J (2008) Aggregation of Ionic Liquids CnMeImBr (n ) 4, 6, 8, 10, 12) in D2O: a NMR study. J Phys Chem B 112:2031–2039
Baker GA, Pandey S, Pandey S, Baker S (2004) A new class of cationic surfactants inspired by N-alkyl-N-methyl pyrrolidinium ionic liquids. Analyst 129:890–892
Jones MJ, Chapman D (1995) Micelles, monolayers, and biomembranes. Wiley-LISS, New York
Holmberg K, Jonsson B, Kronberg B, Lindman B (2003) Surfactants and Polymers in aqueous solution. John Wiley & Sons Ltd, New York
Tadros TF (2005) Applied Surfactants, Principles and Applications . Wiley-VCH Verlag GmbH & Co, KGaA-Weinheim
Nusselder JJH, Engberts JBFN (1992) Toward a better understanding of the driving force for micelle formation and micellar growth. J Colloid Interface Sci 148:353–361
Sirieix-Plenet J, Gaillon L, Letellier P (2004) Behaviour of a binary solvent mixture constituted by an amphiphilic ionic liquid, 1-decyl-3-methylimidazolium bromide and water: Potentiometric and conductimetric studies. Talanta 63:979–986
Turro NJ, Yekta A (1978) Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles. J Am Chem Soc 100:5951–5952
Dong DC, Winnik MA (1982) Photochem. The Py scale of solvent polarities. Solvent effects on the vibronic fine structure of pyrene fluorescence and empirical correlations with ET and Y values. Photobiol 35:17–21
Wang GJ, Engberts JBFN (1994) Induction of aggregate formation of cationic polysoaps and surfactants by low concentrations of additives in aqueous solution. Langmuir 10:2583–2587
Quadrifoglio F, Crescenzi V (1971) The interaction of methyl orange and other azo-dyes with polyelectrolytes and with colloidal electrolytes in dilute aqueous solution. J Colloid Interface Sci 35:447–459
Dutta RK, Bhat SN (1993) interaction of methyl orange with submicellar cationic surfactants. Bull Chem Soc Jpn 66:2457–2460
Dutta RK, Bhat SN (1996) Interaction of phenazinium dyes and methyl orange with micelles of various charge types. Colloids Surf A Physicochem Eng Asp 106:127–134
Buwalda RT, Jonker JM, Engberts JBFN (1999) Aggregation of azo dyes with cationic amphiphiles at low concentrations in aqueous solution. Langmuir 15:1083–1089
Karukstis KK, Savin DA, Loftus CT, Angelo NDD (1998) Spectroscopic studies of the interaction of methyl orange with cationic alkyltrimethylammonium bromide surfactants. J Colloid Interface Sci 203:157–163
Lueck HB, Rice BL, McHale JL (1992) Aggregation of triphenylmethane dyes in aqueous solution: Dimerization and trimerization of crystal violet and ethyl violet. Spectrochim Acta A 48:819–828
Lueck HB, Mchale JL, Edwards WD (1992) Symmetry-breaking solvent effects on the electronic structure and spectra of a series of triphenylmethane dyes. J Am Chem Soc 114:2342–2348
Acknowledgments
We thank FAPESP (São Paulo State Research Foundation) for financial support of this work and a PD fellowship to N. I. Malek; CNPq (National Council for Scientific and Technological Research) for a research productivity fellowship to O. A. El Seoud, Maulana Azad National Fellowship (MANF-2012-13-MUS-GUJ-10818) for a research fellowship to Z. Vaid, and TEQIP fellowship to U. More.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 230 kb).
Rights and permissions
About this article
Cite this article
Malek, N.I., Vaid, Z.S., More, U.U. et al. Ionic-liquid-based surfactants with unsaturated head group: synthesis and micellar properties of 1-(n-alkyl)-3-vinylimidazolium bromides. Colloid Polym Sci 293, 3213–3224 (2015). https://doi.org/10.1007/s00396-015-3746-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3746-x