Abstract
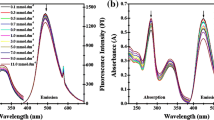
This study attempts to explain the bioavailability, permeability, and interaction parameters of organotin(IV) derivatives with the cell membrane. For this purpose, tributylstannic[3-(3′,4′-dichlorophenylamido)propanoate] was synthesized and characterized by elemental analysis and spectroscopic methods (Fourier transform infrared spectroscopy (FT-IR), 1H, 13C, 119Sn NMR). The organic anion was found to act as monodentate O-bound ligand in solution. Cationic surfactant cetyl N,N,N-trimethylammoniumbromide (CTAB) was used to study the interactions of the organotin(IV) complex with positively charged molecules of the surfactant acting as a model cell membrane. The thermodynamic parameters of complex–CTAB interaction concluded that the complex is located in the peripheral region of CTAB micelles. The redshifts in the UV spectra of the complex confirmed its solubilization into micelles. The two carbonyl oxygen atoms were found to be binding sites of the complex with CTAB.






Similar content being viewed by others
References
Tabassum S, Pettinari C (2006) Chemical and biotechnological developments in organotin cancer chemotherapy. J Organomet Chem 691(8):1761–1766. doi:10.1016/j.jorganchem.2005.12.033
Gielen M, Biesemans M, Willem R (2005) Organotin compounds: from kinetics to stereochemistry and antitumour activities. Appl Organomet Chem 19(4):440–450. doi:10.1002/aoc.771
Ashfaq M, Khan MI, Baloch MK, Malik A (2004) Biologically potent organotin(IV) complexes of 2-maleimidoacetic acid. J Organomet Chem 689(1):238–245. doi:10.1016/j.jorganchem.2003.10.007
Kaluđerović GN, Paschke R, Prashar S, Gómez-Ruiz S (2010) Synthesis, characterization and biological studies of 1-D polymeric triphenyltin(IV) carboxylates. J Organomet Chem 695(15–16):1883–1890. doi:10.1016/j.jorganchem.2010.04.029
Khan MI, Kaleem Baloch M, Ashfaq M (2004) Biological aspects of new organotin(IV) compounds of 3-maleimidopropionic acid. J Organomet Chem 689(21):3370–3378. doi:10.1016/j.jorganchem.2004.07.049
Chicano JJ, Ortiz A, Teruel JA, Aranda FJ (2001) Organotin compounds alter the physical organization of phosphatidylcholine membranes. Biochim Biophys Acta 1510(1–2):330–341. doi:10.1016/S0005-2736(00)00365-5
Kim DH, Lee SK, Kim WS, Yang JS, Lee DW (2001) Quantitative retention activity relationship of quinolones using micellar liquid chromatography. J Liq Chromatogr Relat Technol 24(9):1309–1322. doi:10.1081/JLC-100103449
Shah FA, Fatima K, Sabir S, Ali S, Qadri I (2014) Tributyltin(IV)[3-(3′,5′-dimethylphenylamido)propanoate] as a potent HCV inhibitor, its delivery study, controlled release and binding sites using CTAB as a standard cell membrane. Appl Organomet Chem 28(2):74–80. doi:10.1002/aoc.3079
Armarego WL, Chai CLL (2013) Purification of laboratory chemicals. Butterworth-Heinemann
Shah FA, Tahir MN, Ali S, Ahmed S, Danish M (2009) 3-[(3,4-Dichlorophenyl)aminocarbonyl]propionic acid. Acta Crystallogr Sect E 65(5):o1130. doi:10.1107/S1600536809015025
Rehman W, Baloch MK, Badshah A (2005) Comparative study of structure: activity relationship of di and triorganotin (IV) complexes of monomethyl glutarate. J Braz Chem Soc 16:827–834
Mahmood S, Ali S, Bhatti M, Shahid K, Shahzadi S, Mazhar M, Khan K, Maharvi G (2004) Synthetic, spectroscopic, thermal and biological studies of tri-, di-and chlorodiorganotin (IV) 2-(4-isobutylphenyl) propanoate. J Chem Soc Pak 26(1):61–68
Tariq M, Muhammad N, Sirajuddin M, Ali S, Shah NA, Khalid N, Tahir MN, Khan MR (2013) Synthesis, spectroscopic characterization, X-ray structures, biological screenings, DNA interaction study and catalytic activity of organotin(IV) 3-(4-flourophenyl)-2-methylacrylic acid derivatives. J Organomet Chem 723:79–89. doi:10.1016/j.jorganchem.2012.09.011
Dakternieks D, Duthie A, Smyth DR, Stapleton CPD, Tiekink ERT (2003) Steric control over molecular structure and supramolecular association exerted by tin- and ligand-bound groups in diorganotin carboxylates. Organometallics 22 (22):4599–4603. doi:10.1021/om0340140
Willem R, Verbruggen I, Gielen M, Biesemans M, Mahieu B, Basu Baul TS, Tiekink ERT (1998) Correlating Mössbauer and solution- and solid-state 117Sn NMR data with X-ray diffraction structural data of triorganotin 2-[(E)-2-(2-hydroxy-5-methylphenyl)-1-diazenyl]benzoates. Organometallics 17(26):5758–5766. doi:10.1021/om980504u
Liu T-Q, Guo R (2006) Influence of low cetyltrimethylammonium bromide concentration on the interactions and properties of hemoglobin with acyclovir. Chin J Chem 24(5):620–626. doi:10.1002/cjoc.200690119
Shah SS, Saeed A, Sharif QM (1999) A study of micellization parameters and electrostatic interactions in micellar solution of sodium dodecyl sulfate. Colloids Surf Physicochem Eng Aspects 155(2–3):405–412. doi:10.1016/S0927-7757(99)00021-7
Farías T, de Ménorval LC, Zajac J, Rivera A (2009) Solubilization of drugs by cationic surfactants micelles: conductivity and 1H NMR experiments. Colloids Surf Physicochem Eng Aspects 345(1–3):51–57. doi:10.1016/j.colsurfa.2009.04.022
Shah SS, Ali Awan M, Ashraf M, Idris SA (1995) Solubilization of phenol and benzyl alcohol by cationic and anionic surfactant micelles. Colloids Surf Physicochem Eng Aspects 105(2–3):319–323. doi:10.1016/0927-7757(95)03335-1
Shah SS, Khan MS, Ullah H, Awan MA (1997) Solubilization of amphiphilic hemicyanine dyes by a cationic surfactant, cetyltrimethylammonium bromide. J Colloid Interface Sci 186(2):382–386. doi:10.1006/jcis.1996.4649
Kawamura H, Manabe M, Miyamoto Y, Fujita Y, Tokunaga S (1989) Partition coefficients of homologous.omega.-phenylalkanols between water and sodium dodecyl sulfate micelles. J Phys Chem 93 (14):5536–5540. doi:10.1021/j100351a042
Valero M, Del Arco-Gómez A, Rodríguez L (2002) New insight into the structure of CTAB micelles in the presence of cyclodextrins, using non-steroidic anti-inflammatory agents – nabumetone, naproxen—as fluorescent probes. J Incl Phenom Macrocycl Chem 42(1–2):121–130. doi:10.1023/A:1014521623774
Park JW, Lee SY, Kim SM (2005) Efficient inclusion complexation and intra-complex excitation energy transfer between aromatic group-modified β-cyclodextrins and a hemicyanine dye. J Photochem Photobiol A: Chem 173(3):271–278. doi:10.1016/j.jphotochem.2005.04.006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, F.A., Khan, A.M., Sabir, S. et al. CTAB–tributylstannic[3-(3′,4′-dichlorophenylamido)propanoate] interaction: a tool for predicting organotin(IV) complex–cell membrane interaction parameters. Colloid Polym Sci 294, 87–94 (2016). https://doi.org/10.1007/s00396-015-3738-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3738-x