Abstract
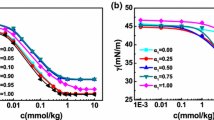
A biphasic solution containing water and precipitated phosphocholine (PC) is presented in order to investigate the consequences of 1,3-butanediol addition on the phase behavior of PC at different 1,3-butanediol concentrations and temperature values. With increasing the concentration of 1,3-butanediol in the mixed solvent at room temperature, the biphasic solution converts into turbid phase, two phase, and finally to a clear phase that is birefringent when viewed between two crossed polarizers. The birefringent phase moves to lower 1,3-butanediol contents at higher temperature values. The birefringent phase was observed via polarizing microscopy, and its rheological parameters were measured with cone plate method. In high 1,3-butanediol contents, solutions with 2.5% of PC behave as a gel with high yield stress value. The micellization of cetyltrimethyammonium bromide (CTAB) in a series of 1,3-butanediol/water mixed solvent at room temperature was also investigated. From the conductivity measurements, the critical micelle concentration and the degree of counterion dissociation of CTAB were obtained as a function of 1,3-butanediol. Standard free energy of micellization, as a function of 1,3-butanediol contents, was also estimated and discussed. Gibbs energies of micellization were found to have a good correlation with dielectric constant and Gordon parameters.











Similar content being viewed by others
References
Seguin C, Eastoe J, Clapperton R, Heenan R, Grillo I (2006) Colloids and Surfaces A: Physicochem. Eng. Aspects 282–283, 134–142
Moyá M, Rodríguez A, Graciani M, Fernández G (2007) J Colloid Interface Sci 316:787
Rodríguez M, Muñoz M, Graciani M, Pachón M, Moyá M (2007) Colloids Surface Physicochem Eng Aspect 298:177
Seguin C, Eastoe J, Rogers S, Hollamby M, Dalgliesh R (2006) Langmuir 22:11187
Seguin C, Eastoe J, Heenan R, Grillo I (2007) Langmuir 23:4199
Hollamby M, Tabor R, Mutch K, Trickett K, Eastoe J, Heenan R, Grillo I (2008) Langmuir 24:12235
Salzer F, Weber G (1950) Zeitschrift für Lebensmitteluntersuchung und -Forschung A 91:174
Wilfried P (2005) Directory of microbicides for the protection of materials. Springer, Dordrecht, pp 263–266, Part one
Shinto K, Hoffmann H, Watanabe K, Teshigawara T (2012) Colloid Polym Sci 290:91
Abdel-Rahem R (2011) J Dispers Sci Technol 32:784
Petrucci R, Harwood W, Herring G, Madura J (2007) General chemistry: principles and modern applications. Prentice Hall, Upper Saddle River
Anton N, Saulnier P, Bduneau A, Benoit J (2007) J Phys Chem B 111:3651
Piekarski H, Jóźwiak M, Woźnicka J, Bald A, Szejgis A (1995) Phys Chem Liq 30:195
Cohen B, Huppert D, Agmon N (2001) J Phys Chem A 105:7165
Cohen B, Huppert D (2000) J Am Chem Soc 122:9838
D’Errico G, Ciccarelli D, Ortona O (2005) J Colloid Interface Sci 286:747
Stuart M, Van de Pas J, Engberts J (2006) J Surfactant and Detergents 9:153
Song A, Reizlein K, Hoffmann H (2008) Progr Colloid Polyme Sci 134:111
Zou A, Hoffmann H, Freiberger N, Glatter O, Makorsky A, Talmon Y (2008) Colloid Surface Physicochem Eng Aspect 316:226
Yan Y, Hoffmann H, Makarsky A, Richter W, Talmon Y (2007) J Phys Chem B 111:6374
Hoffmann H, Löbl M, Rehage H, Wunderlich I (1985) Tenside and Detergents 22:290
Hoffmann H, Thunig C, Schmeidel P, Munkert V, Ulbricht W (1994) Tenside Surf Det 3:389
Din KU, Siddiqui US, Kumar S, Dar A (2006) Colloid Polym Sci 284:807
Ruiz C, Díaz-López L, Aguiar J (2007) J Colloid Interface Sci 305:293
Baloch M, Abdur Rauf F, Durani F, Gulzar H (2010) J Appl Polym Sci 116:2133
Attwood D, Florence A (1985) Surfactant systems: their chemistry, pharmacy, and biology. Chapman & Hall, Norwell
Holland P, Rubingh D (1992) Mixed surfactants system. ASC Symposium Series 501. American Chemical Society, Washington, DC
Evans H (1956) J Chem Soc 579–586
Sjoeberg M, Henriksson U, Waernheim T (1990) Langmuir 6:1205
Hildebrand J, Scott R (1964) Solubility of non-electrolytes. Reinhold/Dover, New York
Kabir-ud-Din A, Koya P (2010) Langmuir 26:7905
Acknowledgments
The author acknowledges Prof. Dr. Heinz Hoffmann for bringing the idea of using 1,3-butanediol in these combinations and also for his useful notes and discussions. Prof. Dr. Grehard Platz is also acknowledged for his kind help in polarization microscopy measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Rahem, R.A. 1,3-Butanediol as a co-solvent for the surfactant solutions. Colloid Polym Sci 290, 907–917 (2012). https://doi.org/10.1007/s00396-012-2603-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-012-2603-4