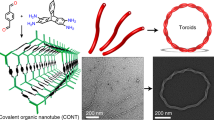
Abstract
We present novel non-symmetric bis-acylurea organogelators that self-assemble into hollow tubular nanostructures upon cooling in solutions. The bis-acylureas have aliphatic end groups of different lengths divided by a spacer group [−NHCONHCO−(CH2)5−CONHCONH−, C5] with two hydrogen bonding sites. Due to the intermolecular biaxial hydrogen bonding, the molecules crystallize into 2D thin layers at first, and then their wrapping ultimately results in nanotubes. On the contrary, symmetric bis-acylureas form multilayered nanosheets which are stabilized by the van der Waals interaction between the stacked layers. The size and shape of the nanotubes can be controlled by varying the difference of the alkyl chain lengths. When the difference is big, for example, eight methylene units (BuC5DD, butyl (Bu) and dodecyl (DD)), uniform nanotubes of 65-nm mean outer diameter are obtained, while a non-symmetric bis-acylurea with one methylene unit difference (UDC5DD, undecyl (UD)) forms a mixture of nanosheets and nanotubes. Template-assisted formation of nanotubes was successfully performed via gelation in inorganic nanopores. We also synthesized a thiol-functionalized non-symmetric bis-acylurea, HS-UDC5Bu (thiol (HS)), which was used as a tubular template for gold nanoparticles.










Similar content being viewed by others
References
Ghadiri MR, Granja JR, Milligan RA, McRee DE, Khazanovich N (1993) Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 366:324–327
Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI (2008) Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science 319(5871):1812–1816
Shao H, Parquette JR (2009) Controllable peptide-dendron self-assembly: interconversion of nanotubes and fibrillar nanostructures. Angew Chem Int Ed 48(14):2525–2528
Lee HY, Nam SR, Hong JI (2007) Microtubule formation using two-component gel system. J Am Chem Soc 129(5):1040–1041
Kavallaris M (2010) Microtubules and resistance to tubulin-binding agents. Nature Rev Cancer 10:194–204
Kerssemakers JWJ, Munteanu EL, Laan L, Noetzel TL, Janson ME, Dogterom M (2006) Assembly dynamics of microtubules at molecular resolution. Nature 442:709–712
Kikkawa M, Metlagel Z (2006) A molecular “zipper” for microtubules. Cell 127(7):1302–1304
Bethune DS, Kiang CH, de Vries MS, Gorman G, Savoy R, Vazquez J, Beyers R (1993) Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 363:605–607
Banerjee S, Hemraj-Benny T, Wong SS (2005) Covalent surface chemistry of single-walled carbon nanotubes. Adv Mater 17(1):17–29
Tasis D, Tagmatarchis N, Bianco A, Prato M (2006) Chemistry of carbon nanotube. Chem Rev 106(3):1105–1136
Steinhard M, Wehrspohn RB, Goesele U, Wendorff JH (2004) Nanotubes by template wetting: a modular assembly system. Angew Chem Int Ed 43(11):1334–1344
Shimizu T, Masuda M, Minamikawa H (2005) Supramolecular nanotube architectures based on amphiphilic nolecules. Chem Rev 105(4):1401–1444
Terech P, De Geyer A, Struth B, Talmon Y (2002) Self-assembled monodisperse steroid nanotubes in water. Adv Mater 14(7):4495–498
Diaz N, Simon FX, Schmutz M, Rawiso M, Decher G, Jestin J, Mesini PJ (2005) Self-assembled diamide nanotubes in organic solvents. Angew Chem Int Ed 44(21):3260–3264
Lee E, Kim JK, Lee M (2009) Reversible scrolling of 2D sheets from self-assembly of laterally-grafted amphiphilic rods. Angew Chem Int Ed 48(20):3657–3660
Khanna S, Khan MK, Sundararajan P (2009) Influence of double hydrogen bonds and alkyl chains on the gelation of nonchiral polyurethane model compounds: sheets, eaves trough, tubes and oriented fibers. Langmuir 25(22):13183–13193
Fuhrhop JH, Schnieder P, Boekema E, Helfrich W (1988) Lipid bilayer fibers from diastereomeric and enantiomeric N-octylaldonamides. J Am Chem Soc 110(9):2861–2867
Pakhomov S, Hammer RP, Mishra BK, Thomas BN (2003) Chiral tubule self-assembly from an achiral diynoic lipid. Proc Natl Acad Sci USA 100(6):3040–3042
Davis R, Berger R, Zentel R (2007) Two-dimensional aggregation of organogelators induced by biaxial hydrogen-bonding gives supramolecular nanosheets. Adv Mater 19(22):3878–3881
Mallia VA, George M, Blair DL, Weiss RG (2009) Robust organogels from nitrogen-containing derivatives of (R)-12-hydroxystearic acid as gelators: comparisons with gels from stearic acid derivatives. Langmuir 25(15):8615–8625
Fages F (2005) Systematic design of amide- and urea-type gelators with tailored properties. In: Low molecular mass gelators: topics in current chemistry, chapter 3. Springer, Berlin
van Esch J, Schoonbeek F, de Loos M, Kooijman H, Spek AL, Kellogg RM, Feringa BL (1999) Cyclic bis-urea compounds as gelators for organic solvents. Chem Eur J 5(3):937–950
Dawn A, Fujita N, Haraguchi S, Sada K, Shinkai S (2009) An organogel system can control the stereochemical course of anthracene photodimerization. Chem Commun (16):2100–2102
Helfrich W, Prost J (1988) Intrinsic bending force in anisotropic membranes made of chiral molecules. Phys Rev A 38(6):3065–3068
Radzihovsky L, Toner J (1995) A new phase of tethered membranes: tubules. Phys Rev Lett 75(26):4752–4755
Bowick M, Falcioni M, Thorleifsson G (1997) Numerical observation of a tubular phase in anisotropic membranes. Phys Rev Lett 79(5):885–888
Dasgupta D, Kamar Z, Rochas C, Dahmani M, Mesini P, Guenet JM (2010) Design of hybrid networks by sheathing polymer fibrils with self-assembled nanotubules. Soft Matter 6(15):3573–3581
Gao X, Matsui H (2005) Peptide-based nanotubes and their applications in bionanotechnology. Adv Mater 17(17):2037–2050
Cui H, Muraoka T, Gheetham AG, Stupp SI (2009) Self-assembly of giant peptide nanobelts. Nano Lett 9(3):945–951
Freeman RG, Grabar KC et al (1995) Self-assembled metal colloid monolayers: an approach to SERS substrates. Science 267(5204):1629–1632
Matsui J, Akamatsu K et al (2005) SPR sensor chip for detection of small molecules using molecularly imprinted polymer with embedded gold nanoparticles. Anal Chem 77(13):4282–4285
Scodeller P, Flexer V et al (2008) Wired-enzyme core–shell Au nanoparticle biosensor. J Am Chem Soc 130(38):12690–12697
Bunz UHF, Rotello VM (2010) Gold nanoparticle–fluorophore complexes: sensitive and discerning “noses” for biosystems sensing. Angew Chem Int Ed 49(19):3268–3279
Acknowledgment
We thank DFG (IRTG1404) for financial support. We also thank the Department of Electron Microscopy at the MPI-P in Mainz for providing support for SEM measurements and P. J. Roth for helpful discussion.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
The experimental details (DOC 312 kb)
Rights and permissions
About this article
Cite this article
Kim, JU., Haberkorn, N., Theato, P. et al. Controlled fabrication of organic nanotubes via self-assembly of non-symmetric bis-acylurea. Colloid Polym Sci 289, 1855–1862 (2011). https://doi.org/10.1007/s00396-011-2512-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-011-2512-y